Figure 7. Foxo3-deficiency in T cells is associated with reduced differentiation of IFN-γ and GM-CSF pathogenic CD4+ T cells during EAE.
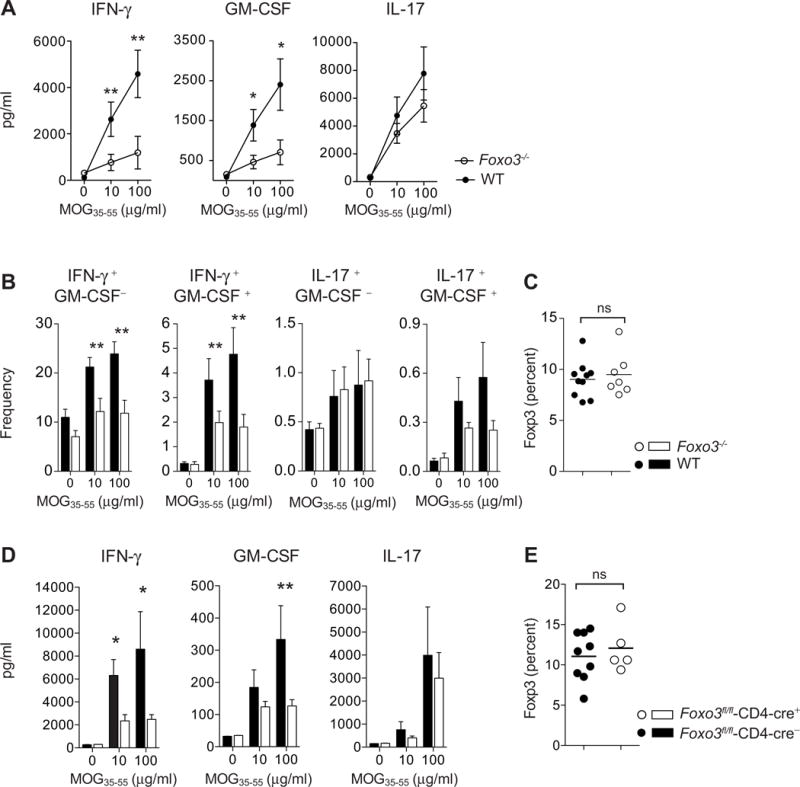
(A) Foxo3−/− (open circles, n=8) and WT littermate mice (black circles, n=8) were immunized with 50μg of peptide MOG35–55 emulsified in CFA. At day 9 post-immunization, CD4+ T cells were purified from spleens and restimulated in vitro with WT APC and MOG35–55 peptide: The secretion of IFN-γ, GM-CSF and IL-17 was analyzed by ELISA in the supernatant after 3 days of culture (n=4 mice per genotype) (B) Frequency of IFN-γ, GM-CSF and IL-17 producing CD4+ T cells was determined by intracellular staining after overnight restimulation with MOG35–55 peptide (n=8 mice per genotype). (C) The expression of Foxp3 by splenic CD4+ T cells from WT and Foxo3−/− mice was assessed by intracellular staining (n=8 mice per genotype) (D) Foxo3fl/fl-Cd4-cre+ or Foxo3fl/fl-Cd4-cre− littermate controls were immunized with 100μg of peptide MOG35–55 emulsified in CFA. At day 9 post-immunization, splenocytes were restimulated in vitro with MOG35–55 peptide and IFN-γ, GM-CSF and IL-17 secretion was analyzed by ELISA (n=9 mice per genotype). (E) The expression of Foxp3 by splenic CD4+ T cells from Foxo3fl/fl-Cd4-cre+ or Foxo3fl/fl-Cd4-cre− mice was assessed. (n=8 mice per genotype). Data are representative of at least two independent experiments. Error bars, s.e.m.; P values (Mann–Whitney U test). See also Figure S7
