Abstract
Bicuspid aortic valve (BAV) is the most common congenital heart defect and falls in the spectrum of left-sided heart defects, also known as left ventricular outflow tract obstructive (LVOTO) defects. BAV is often identified in otherwise healthy, asymptomatic individuals, but it is associated with serious long term health risks including progressive aortic valve disease (stenosis or regurgitation) and thoracic aortic aneurysm and dissection. BAV and other LVOTO defects have high heritability. Although recommendations for cardiac screening of BAV in at-risk relatives exist, there are no standard guidelines for providing genetic counseling to patients and families with BAV. This review describes current knowledge of BAV and associated aortopathy and provides guidance to genetic counselors involved in the care of patients and families with these malformations. The heritability of BAV and recommendations for screening are highlighted. While this review focuses specifically on BAV, the principles are applicable to counseling needs for other LVOTO defects.
Keywords: Congenital heart defect, Left ventricular outflow tract obstruction, LVOTO, Aortic aneurysm, Aortic dissection, Genetic counseling
Introduction
Bicuspid aortic valve (BAV) is the most common congenital heart defect (CHD) with an estimated prevalence of 0.5–2 % in the general population (Braverman et al. 2005; Roberts 1970; Steinberger et al. 2000). BAV describes an aortic valve with two leaflets instead of the normal three. It is a phenotypically heterogeneous malformation with significant variability in fusion patterns (Angelini et al. 1989). There is evidence that BAV results from abnormal fusion during embryonic development (Sans-Coma et al. 1996), but knowledge regarding the specific initiating mechanisms is incomplete. For an extensive review of embryology, morphology, medical management, and imaging information beyond the scope of this review, refer to Braverman et al. (2005).
BAV falls in the spectrum of left-sided heart defects, also known as left ventricular outflow tract obstructive (LVOTO) defects. LVOTO defects comprise a class of cardiac malformations typified by stenotic lesions beginning in the left heart chamber extending out to the descending aortic arch. The LVOTO spectrum includes: mitral valve abnormalities, aortic stenosis (valvar, subvalvar, and supravalvar), BAV, coarctation of the aorta, Shone’s complex and hypoplastic left heart (HLHS) (Aboulhosn and Child 2006; McBride et al. 2005). LVOTO defects account for 15–20 % of all CHDs and have wide clinical and anatomic heterogeneity.
BAV is often identified incidentally in otherwise healthy, asymptomatic patients, but it is associated with serious long term health risks. For example, aortic valve and ascending aortic complications occur in approximately 35 % of individuals, many of whom require life-saving surgical management (Lewin and Otto 2005; Vallely et al. 2008; Ward 2000). Given its prevalence, and the fact that significant complications occur in over one-third of cases, BAV has high morbidity (Siu and Silversides 2010; Ward 2000). BAV often co-occurs with other CHDs and >50 % of people with coarctation of the aorta have BAV (Roos-Hesselink et al. 2003).
While BAV is often found in non-syndromic individuals, it also can be a feature of connective tissue disorders (e.g. Marfan, Loeys-Dietz, and vascular Ehlers-Danlos syndromes) and other syndromes such as Turner and Williams syndromes. BAV was historically assumed to be a sporadic malformation, but reports of familial clustering (Brown et al. 2003; Gale et al. 1977) are consistent with an autosomal dominant inheritance pattern with incomplete penetrance and variable expressivity (Braverman et al. 2005; Huntington et al. 1997). Both BAV and other LVOTO defects have relatively high heritability estimates (Hinton et al. 2007; McBride et al. 2005). Because of the recognition of increased heritability, consensus guidelines recommend the screening of first-degree relatives (Nishimura et al. 2014).
An important goal of cardiovascular genetics is to identify at-risk asymptomatic family members through screening in order to reduce the risk of morbidity and mortality. To date, there is a gap in the genetic counseling literature related to BAV. In this review, we highlight the genetics, heritability, and family screening recommendations for BAV and other LVOTO defects. Our goal is to introduce these concepts to a wider audience and advocate for family screening for all cases of BAV (and LVOTO defects).
BAV, LVOTO, and Heritability
Heritability estimates for BAV and other LVOTO defects range from approximately 0.7 to 0.9, indicating a strong genetic component to the development of this malformation (Cripe et al. 2004; Glick and Roberts 1994; Hinton et al. 2007; Huntington et al. 1997; Lewin et al. 2004; Lewin and Otto 2005; McBride et al. 2005).
When LVOTO defects are identified in a proband, the likelihood of finding LVOTO spectrum defects, including BAV, in family members is high, and recurrence risk of the same cardiovascular malformation or other LVOTO defects ranges from 8 to 22 %, depending on the lesion present in the proband (Hinton et al. 2007; McBride et al. 2005). In a recent study of 249 first-degree relatives of patients with an LVOTO defect, 8 % had a cardiac defect; of these, 68 % had BAV (Kerstjens-Frederikse et al. 2011). More specifically, when BAV is identified in a proband, screening identifies BAV in a first-degree family member in 9 % of these individuals (Huntington et al. 1997). Additionally, BAV has been observed in case reports of monozygotic twins (Brown et al. 2003; Godden et al. 1987).
BAV Associations and Aortic Aneurysm/Dissection
There is an increased risk of thoracic aortic aneurysm and dissection (TAAD) in patients with BAV relative to those with three-leaflet aortic valves. As many as 75 % or more of patients with BAV will develop thoracic aortic aneurysm (TAA) (Tadros et al. 2009). Mild ascending aortic dilatation presenting in middle to older age is the most common dilation pattern seen in patients with BAV. Although less common, aortic root dilatation in the presence of BAV can be seen and has been correlated with younger age of onset and male sex (Della Corte et al. 2007). There is likely a developmental basis for this association, which may be related to a common neural crest cell defect as the cusps of the aortic valve and arterial media of the aortic arch are derived from neural crest cells (Hinton 2012; Schievink and Mokri 1995). Michelena et al. (2008) found that among 212 young patients with normally functioning or minimally dysfunctional BAV, 30 % developed TAA over a mean follow-up period of 10 years. Co-existence of TAA and normally functioning BAV supports the theory that developmental mechanisms are more significant than hemodynamic disturbances, but the precise mechanisms have not been established. In relatives of patients with BAV, ascending aortic dilation was observed in 35 % of individuals with or without BAV (Loscalzo et al. 2007). Biner et al. (2009), documented that aortic root dilation is relatively prevalent (32 %) in first-degree relatives of BAV patients. Again, aortic root dilation was also present in relatives with three-leaflet aortic valve morphology. These observations are consistent with the theory that BAV and TAA are both manifestations of a common developmental abnormality of the aorta (Biner et al. 2009).
TAA in the presence of BAV poses a risk for aortic dissection. In an early study, BAV was associated with approximately 6 % lifetime risk of thoracic aortic dissection, a nine-fold increased risk compared to the general population (Edwards et al. 1978). Increased risk of dissection associated with BAV may be due to higher prevalence and rate of aortic dilation, which occurs at a significantly younger age than idiopathic TAA (Davies et al. 2007; Edwards et al. 1978; La Canna et al. 2006). Long-term cardiac care aims to prevent TAA progression and dissection using medical therapy, such as beta blockers, and exercise and activity restrictions. Progression of aortic dilation and valve function are serially monitored with cardiac imaging modalities such as echocardiography, computerized tomography (CT scan) and/or cardiac magnetic resonance imaging (MRI). There are established guidelines recommending aortic size thresholds for prophylactic aortic replacement surgery in asymptomatic patients indicating that aortic replacement is warranted in patients with BAV when the aortic diameter is 5.5 cm or greater (Hiratzka et al. 2010). However, there has been recent discussion of whether aortic replacement should be performed simultaneously in patients with BAV and smaller aortic diameters who are already undergoing aortic valve replacement or who have additional risk factors such as a rapidly dilating aorta (>0.5 cm per year) or family history of aortic dissection (Hiratzka et al. 2016).
Genetic Counseling and Family Screening Recommendations
Genetic counseling is an essential component of the genetic evaluation for patients with CHDs, including BAV and other left-sided heart defects. Genetic counseling helps patients understand the developmental basis of their cardiac defect(s), screening recommendations for themselves and other family members, recurrence risks, and extra-cardiac features of disease that may require additional management and/or treatment. Additionally, genetic counselors can provide psychosocial support and resources to patients and families with CHDs. As roles of genetic counselors continue to expand, these professionals should be seen as valuable assets in comprehensive care models for the CHD patient population.
In the case of non-syndromic BAV and other LVOTO defects, guidelines are available that endorse family screening. The 2010 American College of Cardiology/American Heart Association (ACC/AHA) Guidelines for the Management of Patients with Thoracic Aortic Disease recommends (Class 1) that all:
First-degree relatives of patients with a bicuspid aortic valve, premature onset of thoracic aortic disease with minimal risk factors, and/or familial form of thoracic aortic aneurysm and dissection should be evaluated for the presence of a bicuspid aortic valve and asymptomatic thoracic aortic disease
(Hiratzka et al. 2010) (p. 1555).
This recommendation is based on the principle that detection of sub-clinical BAV allows for additional observation and intervention that could prevent long-term complications associated with aortic stenosis, aortic aneurysm, and dissection. Specific intervals at which this screening should occur to address the risk of BAV-associated aortic dilation have not been delineated in the recommendations.
Echocardiography has excellent sensitivity detecting 92–96 % of cases of BAV (Siu and Silversides 2010) while physical exam alone only detects 50 % of cases (Nistri et al. 2005). These guidelines are even more pertinent considering studies by Biner et al. (2009) and Loscalzo et al. (2007) showing that aortic aneurysm can be identified in family members of BAV patients (even though they might have normal three-leaflet valve morphology). There is also an increased incidence of BAV in relatives of patients with other LVOTO defects, and screening of first-degree family members has been recommended in these cases (Kerstjens-Frederikse et al. 2011; McBride et al. 2005).
Recurrence risk estimates can be of particular concern for families of patients with CHD when considering future family planning. Recurrence risk for LVOTO defects in first-degree family members can be stratified by taking lesion and relation to the affected family member into consideration (Cowan and Ware 2015). Confirmation of syndromic vs. non-syndromic disease can also help to refine recurrence risk. Non-syndromic BAV has been reported to have a recurrence risk ranging from 1 to 5 % for first-degree family members (Swain 2011). Others have estimated this risk as high as 9 % (Huntington et al. 1997). A more recent study found a recurrence risk in the siblings of BAV probands to be 10.1 % (Hales and Mahle 2014). Cowan and Ware (2015) include a list of recurrence risk for various CHDs, including known data for LVOTO defects (Cowan and Ware 2015). However, specific recurrence risk data may not be available for some lesions. Recording a thorough three- to four-generation pedigree with specific focus on LVOTO defects will also help to elucidate the specific risks to the family member(s) in question. The increased risk of BAV and LVOTO defects in other family members should also be discussed in conjunction with relevant first-degree family screening recommendations.
Special consideration should be taken when counseling a woman with BAV in a prenatal setting. The ACC/AHA adult congenital heart disease guidelines, valvar heart disease guidelines and European Society of Cardiology guidelines recommend preconception counseling and discussion of contraceptive options for women with BAV. During pregnancy, changes in hemodynamics as well as changes in the aortic media, put women with BAV with or without significant aortic stenosis and/or dilated aortic roots at risk for complications. Potential risks that should be discussed include progressive aortic enlargement or dissection, and complications of aortic stenosis and/or aortic regurgitation, heart failure or delivery complications. Echocardiogram before and during pregnancy to monitor ascending aorta dilation is recommended (Hiratzka et al. 2010 ACC/AHA Guidelines) as well as consideration for prophylactic aortic repair if the aortic diameter is >4.0 cm for women planning a future pregnancy. Although the risk for complication is low during pregnancy for women with BAV, they should still be counseled regarding potential risks and treatment prior to and during pregnancy (McKellar et al. 2011).
Molecular Genetics of BAV: Implications for Genetic Testing
Cardiac valve morphogenesis is a process involving multiple signaling pathways including, but not limited to, members of the TGF-β superfamily, VEGF, Notch, Wnt/β-catenin, Tbx20, and Gata4 (Chakraborty et al. 2010; Laforest and Nemer 2012). Several other genes have been implicated in BAV development, including genes involved in connective tissue disorders, cell signaling, and the extra-cellular matrix. It is likely that perturbations in several of these pathways contribute to BAV, and mouse studies are providing insight into new BAV candidate genes (Chakraborty et al. 2010; Laforest and Nemer 2012). For an in-depth review of the molecular genetic basis of BAV development, refer to Padang et al. (2013). Despite this increased understanding of BAV heritability, few genes have been directly linked to BAV development. Martin et al. (2007) determined linkage to three chromosomal regions, 18q, 5q, and 13q. Only NOTCH1, located at 9q34.3, has been associated with non-syndromic BAV in a limited number of familial cases and about 4 % of sporadic cases (Garg et al. 2005; McKellar et al. 2007; Mohamed et al. 2006).
Recently, Pepe et al. (2014) identified FBN1 mutations in two patients with BAV and aortic root dilation with variable Marfan phenotypes. This suggested that some patients with BAV could have FBN1 mutations with a milder connective tissue disorder phenotype warranting evaluation by a clinical geneticist (Pepe et al. 2014). Additionally, a loss-of-function mutation in NKX2–5 (at 5q34) was recently associated with familial, isolated BAV in a Han Chinese family (Qu et al. 2014). Other genes that have been implicated in isolated BAV or BAV with TAAD include ACTA2, TGFB2, SMAD6, and GATA5 (Bonachea et al. 2014; Guo et al. 2007; Laforest and Nemer 2012; Lindsay et al. 2012; Tan et al. 2012). More research is needed to estimate how frequently BAV is associated with variants in these genes. Table 1 lists genes currently known to be associated with syndromic and non-syndromic BAV (with or without TAAD).
Table 1.
Currently known genes contributing to isolated and syndromic BAV
| Gene | Associated Malformations/Syndromes | Phenotype | Reference(s) |
|---|---|---|---|
| NOTCH1 | CHD, BAV | BAV (familial and sporadic), LVOTO | McBride et al. (2008); McKellar et al. (2007); Mohamed et al. (2006); Garg et al. (2005) |
| FBN1 | Marfan syndrome | BAV in Marfan syndrome, possibly isolated BAV (see reference) | Pepe et al. (2014) |
| TGFBR2 | Loeys-Dietz syndrome (type 2), familial TAAD | Hypertelorism, BAV, arterial tortuosity, bifid uvula, CTD features | * See note below |
| TGFB2 | Loeys-Dietz syndrome (type 4) | Hypertelorism, bifid uvula, BAV, pectus, arterial tortuosity, other CTD features | Lindsay et al. (2012) |
| SMAD6 | Aortic valve malformations | BAV, AS, HTN, CoA | Tan et al. (2012) |
| ACTA2 | Familial TAAD | TAAD, BAV | Guo et al. (2007) |
| NKX2–5 | BAV, CHD | BAV, ASD, AS, PFO, other CHD | Qu et al. (2014) |
| GATA5 | AVSD, BAV | Ackerman et al. (2012); Padang et al. (2013) |
Acronyms: AS = aortic stenosis; ASD = atrial septal defect; CoA = coarctation of the aorta; CHD = congenital heart defects; CTD = connective tissue disorder; HTN = hypertension; LVOTO = left ventricular outflow tract obstructions; PFO = patent foramen ovale; TAAD = thoracic aortic aneurysm/dissection;
TGFBR2 is associated with TAAD and Loeys-Dietz syndrome type 2, but BAV is relatively uncommon in this type (see Clinical Synopsis of OMIM entry: http://omim.org/entry/610168)
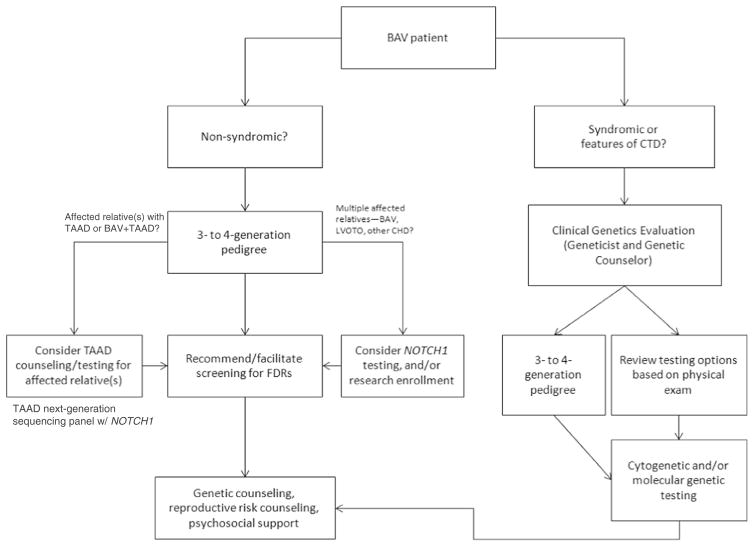
Genetic testing for isolated BAV may not have a high yield even in familial cases, most likely due to an incomplete understanding of BAV development. Foffa et al. (2013) identified mutations in NOTCH1 in a small cohort of 11 patients with familial BAV (defined as 2 or more affected family members) but did not identify mutations in GATA5, TGFBR1 or TGFBR2 (other genes with hypothesized roles in isolated BAV). Likewise, Arrington et al. (2008) did not identify TGFBR1 or TGFBR2 mutations in slightly larger cohort of 35 patients with BAV. More recently, NOTCH1 mutations have been found in 7 % of familial left-sided heart defects compared to 1 % of sporadic cases (Kerstjens-Frederikse et al. 2016). Thus, current clinical genetic testing for apparently isolated BAV has low yield at present, but could be considered for particularly motivated families or those with striking family histories of BAV or other LVOTO defects. The presence of BAV in the patient with aortic dilation should not preclude a provider from ordering genetic testing for TAAD. Several commercial laboratories currently offer next-generation sequencing panels for TAAD which include the NOTCH1, TGFB2, and ACTA2 genes (in addition to other genes associated with TAAD and connective tissue disorders). Mutations in NOTCH1 have been reported in patients with BAV and aortic dilation (McKellar et al. 2007; Proost et al. 2015). Given the relative cost-effectiveness of multi-gene panels and low yield of single gene sequencing, a larger TAAD panel could be considered over single-gene sequencing for patients with BAV and aortic dilation. Figure 1 outlines an algorithm for considering molecular and/or cytogenetic testing for BAV cases.
Fig. 1.
Clinical algorithm for patients with BAV. Acronyms used in this table: CTD = connective tissue disorder(s); TAAD = thoracic aortic aneurysms/dissections; FDRs = first-degree relatives
Last, pre-test genetic counseling is critical for all patients undergoing genetic testing. It has particular importance for individuals with BAV or TAAD who have low to moderate Ghent or Beighton scores for syndromic connective tissue disorders. Discussion with the patient should include the possibility of significant changes to medical management should genetic testing confirm a syndromic diagnosis. Of particular concern are the recommendations for surgical intervention at smaller aortic diameters in certain syndromic conditions as well as additional management or surveillance recommendations.
Discussion
The goal of this review is to provide an overview of the current knowledge about BAV and aortopathy, associated health risks, and updates regarding heritability and family screening recommendations. There is increased recognition of the importance of family history and family screening for BAV and LVOTO defects that is reflected by the recommendation by the ACC/AHA that first-degree family members of BAV patients should be screened for BAV as well (Hiratzka et al. 2010). As outlined in this review, BAV is associated with a number of long-term health risks if not identified early and managed appropriately. Given the lack of clear recommendations as to when cardiac screening should begin or at what intervals should be repeated for BAV and other LVOTO defects, close communication between genetics and cardiology providers will be crucial to developing an individualized care plan for each affected individual and at-risk family members based on family history of specific left-sided lesions (McBride and Garg 2011). The yield of current molecular genetic testing for isolated BAV is low, despite an understanding of its heritability.
Identifying at-risk family members and facilitating family screening is paramount to reducing the long-term morbidity and mortality associated with inherited LVOTOs. Genetic counselors are primed to be key players for addressing these issues given their skills and training to collect and interpret family history and provide family risk assessment. In addition, genetic counselors can address the unique psychosocial concerns present in many families with inherited cardiovascular conditions by promoting psychological well-being through health education and emotional support (Davey et al. 2005).
Given the prevalence of BAV in the general population, the recommendations for health supervision and family screening should not fall only under the purview of the specialist cardiovascular genetic counselor. We would advocate that all genetic counselors, geneticists, cardiologists, or others involved in the care of the cardiogenetics patient should have a working knowledge of the health risks associated with BAV and other LVOTOs as well as the relevant screening recommendations for affected individuals and at risk family members.
Conclusions & Practice Implications
Genetic counselors should be aware of the heritability and family screening recommendations for BAV and be prepared to discuss the following information with patients/families:
High heritability of BAV (and the limited molecular genetic testing options currently available for isolated BAV)
Associations of BAV with other syndromes and connective tissue disorders, recognizing the necessity for evaluation by a clinical geneticist for diagnosis, accurate recurrence risk information, and relevant family screening recommendations
Recommendation for echocardiography screening of first-degree relatives of someone with BAV; refer to Fig. 1 for a proposed clinical algorithm for genetic counselors working with BAV patients
Provision of recurrence risk information: the BAV recurrence risk to first-degree relatives has been previously estimated up to 10 % (Hales and Mahle 2014; Huntington et al. 1997)
Genetic counselors can serve as a bridge between genetics and cardiology providers and should promote communication between specialties regarding the care of the BAV/LVOTO defects patient and family. Genetic counselors can also encourage cardiologists and geneticists alike to rely on published health surveillance guidelines for BAV and LVOTO defects when discussing care plans with affected individuals and at-risk family members (Hiratzka et al. 2010). Conversations with families affected by BAV and other LVOTO defects may prove to be complex depending on the personal/family history as well as the indication for genetic counseling. Patients with BAV provide collaborative opportunities for genetics and cardiology professionals to provide better multidisciplinary care addressing both individual and familial healthcare needs.
Acknowledgments
We would like to thank Katherine Spoonamore, MS, CGC for her leadership and support. This publication was made possible by the Indiana University Health – Indiana University School of Medicine Strategic Research Initiative.
Funding No funding was received for this work.
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflict of interest.
Ethical Approval This article does not contain any studies with human participants performed by any of the authors.
Informed Consent No IRB approval or informed consent was required since this review article does not involve protected health information or animal/human subjects research
References
- Aboulhosn J, Child JS. Left ventricular outflow obstruction: subaortic stenosis, bicuspid aortic valve, supravalvar aortic stenosis, and coarctation of the aorta. Circulation. 2006;114(22):2412–2422. doi: 10.1161/CIRCULATIONAHA.105.592089. [DOI] [PubMed] [Google Scholar]
- Ackerman C, Locke AE, Feingold E, Reshey B, Espana K, Thusberg J, et al. An excess of deleterious variants in VEEGF-A pathway genes in Down-syndrome-associated atrioventricular septal defects. American Journal of Human Genetics. 2012;91(4):646–59. doi: 10.1016/j.ajhg.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini A, Ho SY, Anderson RH, Devine WA, Zuberbuhler JR, Becker AE, et al. The morphology of the normal aortic valve as compared with the aortic valve having two leaflets. The Journal of Thoracic and Cardiovascular Surgery. 1989;98(3):362–367. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2770318. [PubMed] [Google Scholar]
- Arrington CB, Sower CT, Chuckwuk N, Stevens J, Leppert MF, Yetman AT, et al. Absence of TGFBR1 and TGFBR2 mutations in patients with bicuspid aortic valve and aortic dilation. The American Journal of Cardiology. 2008;102(5):629–631. doi: 10.1016/j.amjcard.2008.04.044. [DOI] [PubMed] [Google Scholar]
- Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. Journal of the American College of Cardiology. 2009;53(24):2288–2295. doi: 10.1016/j.jacc.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonachea EM, Change SW, Zender G, LaHaye S, Fitzgerald-Butt S, McBride KL, et al. Rare GATA5 sequence variants identified in individuals with bicuspid aortic valve. Pediatric Research. 2014;76(2):211–216. doi: 10.1038/pr.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman AC, Guven H, Beardslee MA, Makan M, Kates AM, Moon MR. The bicuspid aortic valve. Current Problems in Cardiology. 2005;30(9):470–522. doi: 10.1016/j.cpcardiol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Brown C, Sane DC, Kitzman DW. Bicuspid aortic valves in monozygotic twins. Echocardiography. 2003;20(2):183–184. doi: 10.1046/j.1540-8175.2003.03012.x. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Combs MD, Yutzey KE. Transcriptional regulation of heart valve progenitor cells. Pediatric Cardiology. 2010;31(3):414–421. doi: 10.1007/s00246-009-9616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan JR, Ware SM. Genetics and genetic testing in congenital heart disease. Clinics in Perinatology. 2015;42(2):373–393. doi: 10.1016/j.clp.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. Journal of the American College of Cardiology. 2004;44(1):138–143. doi: 10.1016/j.jacc.2004.03.050. [DOI] [PubMed] [Google Scholar]
- Davey A, Rostant K, Harrop K, Goldblatt J, O’Leary P. Evaluating genetic counseling: client expectations, psychological adjustment and satisfaction with service. Journal of Genetic Counseling. 2005;14(3):197–206. doi: 10.1007/s10897-005-0519-6. [DOI] [PubMed] [Google Scholar]
- Davies RR, Kaple RK, Mandapati D, Gallo A, Botta DM, Jr, Elefteriades JA, et al. Natural history of ascending aortic aneurysms in the setting of an unreplaced bicuspid aortic valve. The Annals of Thoracic Surgery. 2007;83(4):1338–1344. doi: 10.1016/j.athoracsur.2006.10.074. [DOI] [PubMed] [Google Scholar]
- Della Corte A, Bancone C, Quarto C, Dialetto G, Covino FE, Scardone M, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. European Journal of Cardio-Thoracic Surgery. 2007;31(3):397–404. doi: 10.1016/j.ejcts.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Edwards WD, Leaf DS, Edwards JE. Dissecting aortic aneurysm associated with congenital bicuspid aortic valve. Circulation. 1978;57(5):1022–1025. doi: 10.1161/01.cir.57.5.1022. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/639201. [DOI] [PubMed] [Google Scholar]
- Foffa I, Ait Ali L, Panesi P, Mariani M, Festa P, Botto N, et al. Sequencing of NOTCH1, GATA5, TGFBR1 and TGFBR2 genes in familial cases of bicuspid aortic valve. BMC Medical Genetics. 2013;14:44. doi: 10.1186/1471-2350-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale AN, McKusick VA, Hutchins GM, Gott VL. Familial congenital bicuspid aortic valve: secondary calcific aortic stenosis and aortic aneurysm. Chest. 1977;72(5):668–670. doi: 10.1378/chest.72.5.668. [DOI] [PubMed] [Google Scholar]
- Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, et al. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437(7056):270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- Glick BN, Roberts WC. Congenitally bicuspid aortic valve in multiple family members. The American Journal of Cardiology. 1994;73(5):400–404. doi: 10.1016/0002-9149(94)90018-3. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8109558. [DOI] [PubMed] [Google Scholar]
- Godden DJ, Sandhu PS, Kerr F. Stenosed bicuspid aortic valves in twins. European Heart Journal. 1987;8:316–318. doi: 10.1093/oxfordjournals.eurheartj.a062276. [DOI] [PubMed] [Google Scholar]
- Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nature Genetics. 2007;39(12):1488–1493. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- Hales AR, Mahle WT. Echocardiography screening of siblings oh children with bicuspid aortic valve. Pediatrics. 2014;133(5):e1212–e1217. doi: 10.1542/peds.2013-3051. [DOI] [PubMed] [Google Scholar]
- Hinton RB. Bicuspid aortic valve and thoracic aortic aneurysm: three patient populations, two disease phenotypes, and one shared genotype. Cardiology Research and Practice. 2012;2012:926975. doi: 10.1155/2012/926975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton RB, Jr, Martin LJ, Tabangin ME, Mazwi ML, Cripe LH, Benson DW. Hypoplastic left heart syndrome is heritable. Journal of the American College of Cardiology. 2007;50(16):1590–1595. doi: 10.1016/j.jacc.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121(13):e266–e369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- Hiratzka LF, Creager MA, Isselbacher EM, Svensson LG, Nishimura RA, Bonow RO, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Journal of the American College of Cardiology. 2016;67(6):724–731. doi: 10.1016/j.jacc.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Huntington K, Hunter AG, Chan KL. A prospective study to assess the frequency of familial clustering of congenital bicuspid aortic valve. Journal of the American College of Cardiology. 1997;30(7):1809–1812. doi: 10.1016/s0735-1097(97)00372-0. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9385911. [DOI] [PubMed] [Google Scholar]
- Kerstjens-Frederikse WS, Du Marchie SGJ, Ruiter JS. Left ventricular outflow tract obstruction: should cardiac screening be offered to first-degree relatives? (vol 97, pg 1228, 2011) Heart. 2011;97(19):1626–1626. doi: 10.1136/hrt.2010.211433corr1. [DOI] [PubMed] [Google Scholar]
- Kerstjens-Frederikse WS, van de Laar IMBH, Vos YJ, Verhagen JMA, Berger RMF, Lichtenbelt KD, et al. Cardiovascular malformations caused by NOTCH1 mutations do not keep left: data on 428 probands with left-sided CHD and their families. Genetics in Medicine. 2016 doi: 10.1038/gim.2015.193. [DOI] [PubMed] [Google Scholar]
- La Canna G, Ficarra E, Tsagalau E, Nardi M, Morandini A, Chieffo A, et al. Progression rate of ascending aortic dilation in patients with normally functioning bicuspid and tricuspid aortic valves. American Journal of Cardiology. 2006;98(2):249–253. doi: 10.1016/j.amjcard.2006.01.096. [DOI] [PubMed] [Google Scholar]
- Laforest B, Nemer M. Genetic insights into bicuspid aortic valve formation. Cardiology Research and Practice. 2012;2012:180297. doi: 10.1155/2012/180297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin MB, Otto CM. The bicuspid aortic valve: adverse outcomes from infancy to old age. Circulation. 2005;111(7):832–834. doi: 10.1161/01.CIR.0000157137.59691.0B. [DOI] [PubMed] [Google Scholar]
- Lewin MB, McBride KL, Pignatelli R, Fernbach S, Combes A, Menesses A, et al. Echocardiographic evaluation of asymptomatic parental and sibling cardiovascular anomalies associated with congenital left ventricular outflow tract lesions. Pediatrics. 2004;114(3):691–696. doi: 10.1542/peds.2003-0782-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay ME, Schepers D, Bolar NA, Doyle JJ, Gallo E, Fert-Bober J, et al. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nature Genetics. 2012;44(8):922. doi: 10.1038/ng.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscalzo ML, Goh DLM, Loeys B, Kent KC, Spevak PJ, Dietz HC. Familial thoracic aortic dilation and bicommissural aortic valve: A prospective analysis of natural history and inheritance. American Journal of Medical Genetics Part A. 2007;143a(17):1960–1967. doi: 10.1002/ajmg.a.31872. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Ramachandran V, Cripe LH, Hinton RB, Andelfinger G, Tabangin M, et al. Evidence in favor of linkage to human chromosomal regions 18q, 5q, and 13q for bicuspid aortic valve and associated cardiovascular malformations. Human Genetics. 2007;121(2):275–284. doi: 10.1007/s00439-006-0316-9. [DOI] [PubMed] [Google Scholar]
- McBride KL, Garg V. Heredity of bicuspid aortic valve: is family screening indicated? Heart. 2011;97(15):1193–1195. doi: 10.1136/hrt.2011.222489. [DOI] [PubMed] [Google Scholar]
- McBride KL, Pignatelli R, Lewin M, Ho T, Fernbach S, Menesses A, et al. Inheritance analysis of congenital left ventricular outflow tract obstruction malformations: segregation, multiplex relative risk, and heritability. American Journal of Medical Genetics Part A. 2005;134A(2):180–186. doi: 10.1002/ajmg.a.30602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar SH, Tester DJ, Yagubyan M, Majumdar R, Ackerman MJ, Sundt TM. Novel NOTCH1 mutations in patients with bicuspid aortic valve disease and thoracic aortic aneurysms. The Journal of Thoracic and Cardiovascular Surgery. 2007;134:290–296. doi: 10.1016/j.jtcvs.2007.02.041. [DOI] [PubMed] [Google Scholar]
- McKellar SH, MacDonald RJ, Michelena HI, Connolly HM, Sundt TM., 3rd Frequency of cardiovascular events in women with a congenitally bicuspid aortic valve in a single community and effect of pregnancy on events. The American Journal of Cardiology. 2011;107(1):96–99. doi: 10.1016/j.amjcard.2010.08.061. [DOI] [PubMed] [Google Scholar]
- Michelena HI, Desjardins VA, Avierinos JF, Russo A, Nikomo VT, Sundt TM, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation. 2008;117(21):2776–2784. doi: 10.1161/CIRCULATIONAHA.107.740878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed SA, Aherrahrou Z, Liptau H, Erasmi AW, Hagemann C, Wrobel S, et al. Novel missense mutations (p.T596 M and p.P1797H) in NOTCH1 in patients with bicuspid aortic valve. Biochemical and Biophysical Research Communications. 2006;345(4):1460–1465. doi: 10.1016/j.bbrc.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. The Journal of Thoracic and Cardiovascular Surgery. 2014;148(1):e1–e132. doi: 10.1016/j.jtcvs.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Nistri S, Basso C, Marzari C, Mormino P, Thiene G. Frequency of bicuspid aortic valve in young male conscripts by echocardiogram. The American Journal of Cardiology. 2005;96(5):718–721. doi: 10.1016/j.amjcard.2005.04.051. [DOI] [PubMed] [Google Scholar]
- Padang R, Bannon PG, Jeremy R, Richmond DR, Semsarian C, Vallely M, et al. The genetic and molecular basis of bicuspid aortic valve associated with thoracic aortopathy: a link to phenotype heterogeneity. Annals of Cardiothoracic Surgery. 2013;2(1):83–91. doi: 10.3978/j.issn.2225-319X.2012.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe G, Nistri S, Giusti B, Sticchi E, Attanasio M, Porciani C, et al. Identification of fibrillin 1 gene mutations in patients with bicuspid aortic valve (BAV) without Marfan syndrome. BMC Medical Genetics. 2014;15:23. doi: 10.1186/1471-2350-15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proost D, Vandeweyer G, Meester JAN, Salemink S, Kempers M, Ingram C, et al. Performance mutation identification using targeted next-generation sequencing of 14 thoracic aortic aneurysm genes. Human Mutation. 2015;36(8):808–814. doi: 10.1002/humu.22802. [DOI] [PubMed] [Google Scholar]
- Qu XK, Qiu XB, Yuan F, Wang J, Zhao CM, Liu XY, et al. A novel NKX2.5 loss-of-function mutation associated with congenital bicuspid aortic valve. The American Journal of Cardiology. 2014;114(12):1891–1895. doi: 10.1016/j.amjcard.2014.09.028. [DOI] [PubMed] [Google Scholar]
- Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. The American Journal of Cardiology. 1970;26(1):72–83. doi: 10.1016/0002-9149(70)90761-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/5427836. [DOI] [PubMed] [Google Scholar]
- Roos-Hesselink JW, Scholzel BE, Heijdra RJ, Spitaels SE, Meijboom FJ, Boersma E, et al. Aortic valve and aortic arch pathology after coarctation repair. Heart. 2003;89(9):1074–1077. doi: 10.1136/heart.89.9.1074. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12923033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans-Coma V, Fernandez B, Duran AC, Thiene G, Arque JM, Munoz-Chapuli R, Cardo M. Fusion of valve cushions as a key factor in the formation of congenital bicuspid aortic valves in Syrian hamsters. The Anatomical Record. 1996;244(4):490–498. doi: 10.1002/(SICI)1097-0185(199604)244:4<490::AID-AR7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Schievink WI, Mokri B. Familial aorto-cervicocephalic arterial dissections and congenitally bicuspid aortic valve. Stroke. 1995;26(10):1935–1940. doi: 10.1161/01.str.26.10.1935. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7570751. [DOI] [PubMed] [Google Scholar]
- Siu SC, Silversides CK. Bicuspid aortic valve disease. Journal of the American College of Cardiology. 2010;55(25):2789–2800. doi: 10.1016/j.jacc.2009.12.068. [DOI] [PubMed] [Google Scholar]
- Steinberger J, Moller JH, Berry JM, Sinaiko AR. Echocardiographic diagnosis of heart disease in apparently healthy adolescents. Pediatrics. 2000;105(4 Pt 1):815–818. doi: 10.1542/peds.105.4.815. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10742325. [DOI] [PubMed] [Google Scholar]
- Swain S. Evaluation of Recurrence Risks for Left-Sided Cardiac Lesions. UT GSBS Dissertations and Theses (OpenAccess), Paper 152. 2011 Online digitalcommons.library.tmc.edu/cgi/viewcontent.cgi?article=1156&context=utgsbs_dissertations.
- Tadros TM, Klein MD, Shapira OM. Ascending aortic dilatation associated with bicuspid aortic valve: pathophysiology, molecular biology, and clinical implications. Circulation. 2009;119(6):880–890. doi: 10.1161/CIRCULATIONAHA.108.795401. [DOI] [PubMed] [Google Scholar]
- Tan HL, Glen E, Topf A, Hall D, O’Sullivan JJ, Sneddon L, et al. Nonsynonymous variants in the SMAD6 gene predispose to congenital cardiovascular malformation. Human Mutation. 2012;33:720–727. doi: 10.1002/humu.22030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallely MP, Semsarian C, Bannon PG. Management of the ascending aorta in patients with bicuspid aortic valve disease. Heart, Lung & Circulation. 2008;17(5):357–363. doi: 10.1016/j.hlc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Ward C. Clinical significance of the bicuspid aortic valve. Heart. 2000;83(1):81–85. doi: 10.1136/heart.83.1.81. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10618341. [DOI] [PMC free article] [PubMed] [Google Scholar]