Abstract
CCAAT/enhancer-binding proteins, C/EBPα and C/EBPβ, are required for fat cell differentiation and maturation. Previous studies showed that replacement of C/EBPα with C/EBPβ, generating the β/β alleles in the mouse genome, prevents lipid accumulation in white adipose tissue (WAT). In this study, β/β mice lived longer and had higher energy expenditure than their control littermates due to increased WAT energy oxidation. The WAT of β/β mice was enriched with metabolically active, thermogenic mitochondria known for energy burning. The β/β allele exerted its effect through the elevated expression of the G protein α stimulatory subunit (Gαs) in WAT. Gαs, when overexpressed in fat-laden 3T3-L1 cells, stimulated mitochondrial biogenesis similar to that seen in the WAT of β/β mice, and effectively diminished the stored lipid pool.
Keywords: C/EBP, gene replacement, Gαs, WAT adipocyte, mitochondrial biogenesis
At its simplest level, obesity is a disorder of energy balance, where energy intake exceeds energy expenditure; as a consequence, the excess energy is stored in the form of fat in adipocytes. The primary onset mechanisms are dietary and/or genetic (Kopelman 2000). Of those known genetic factors involved in determining adiposity in animals, many are regulatory and exert their effect directly on adipocyte differentiation and development (Rosen et al. 2000; Flier 2004). These factors include CCAAT/enhancer-binding proteins (C/EBPs), SREBP, and PPARγ, whose concerted action during adipogenesis leads to the development of fat-laden mature adipocytes (Rosen et al. 2000).
The C/EBP family consists of five members, of which C/EBPα, C/EBPβ, and C/EBPδ have a profound impact on fat cell differentiation (Yeh et al. 1995). During adipogenesis, C/EBP family functions in a transcriptional cascade, in which the early and transiently expressed C/EBPβ and C/EBPδ activate transcription of PPARγ. PPARγ is then responsible for the expression of C/EBPα (Wu et al. 1996; Rosen et al. 1999). Subsequently, PPARγ and C/EBPα, together with SREBP, work synergistically to transactivate expression of most or all of the genes encoding for factors, such as fatty-acid synthase and adipocyte-specific fatty acid-binding protein aP2, which characterize the fat cell phenotype (Speigelman et al. 1993).
In this study, we used previously manipulated gene knock-in mice, known as β/β mice, in which the C/EBPα gene has been replaced by the C/EBPβ gene (referred here as β/β allele) to study their function in tissues (Chen et al. 2000). β/β mice are lean, and despite markedly reduced fat storage in their fat cells, they do not develop hyperlipidemia or fatty liver, commonly found in the forced leaness that typically causes the liver to take up and store fatty acids when their circulating levels are elevated (Moitra et al. 1998; Shimomura et al. 1998; Chen et al. 2000). We monitored closely the physiology and lifespan of the lean β/β mice and undertook mechanistic studies to understand the effect of β/β allele in energy metabolism. We found that the β/β allele caused an increase in mitochondrial biogenesis only in fat cells of white adipose tissues (WAT), and this WAT specifically enhanced mitochondrial biogenesis was possibly elicited by the markedly elevated expression of G protein α stimulatory subunit (Gαs).
Results and Discussion
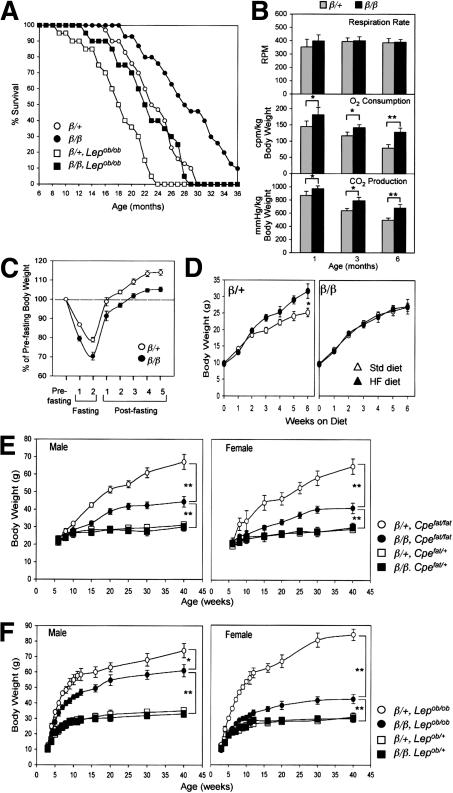
To better understand the physiology of lean β/β mice, we monitored their lifespan, energy expenditure, and responses to diet-induced stress. The average longevity of β/β mice (28.9 ± 5.7 mo; Fig. 1A) was 5.2 mo longer than that of their heterozygous littermates (23.7 + 3.9 mo; Fig. 1A). Although β/β mice consumed more food (Chen et al. 2000), their locomotor activity was similar to that of their heterozygous littermates (data not shown), but their body temperature (38.9°C ± 0.3°C) was 0.3°C-0.5°C higher than that of their wild-type littermates (38.5°C ± 0.2°C). They also consumed and produced significantly more O2 and CO2, respectively, than their heterozygous littermates at the ages examined (Fig. 1B), indicating that β/β mice expend more energy than their heterozygous littermates. To examine their response to dietary stress, β/β mice were fasted or fed a high-fat (HF) diet. Despite losing more of their body weight than their heterozygous littermates during the 48-h fasting period, they recovered it with a speed (Logarithmic trend: y = 19.362Ln(X) + 73.819; R2 = 0.95) similar to that of their heterozygous littermates (Logarithmic trend: y = 19.572Ln(X) + 81.572; R2 = 0.97; Fig. 1C). This indicated that the lean state of β/β mice did not affect their ability to sustain and recover from fasting stress. When fed a high-fat diet (30% fat), the heterozygous control mice gained 25% more weight than those fed the standard diet. In contrast, although β/β mice consumed more HF food (19% more) than their heterozygous littermates throughout the 6-wk period, they did not gain more weight than those fed the standard diet (Fig. 1D), indicating that the β/β mice are protected from diet-induced weight gain.
Figure 1.
State of health of β/β mice and the effect of β/β allele on the monogenic obese mutants Cpefat/fat and Lepob/ob and on weight gain induced by a high-fat diet. (A) Lifespan of β/β mice. The values depicted are the percentage of surviving mice from the original mice of each group (n = 30-40). The respiration parameters (B) and bodyweight recovery after fasting (C) in β/β mice. Each bar represents the mean ± S.E.M. (n = 6-8). (*) p < 0.05; (**) p < 0.01 between the two groups indicated. The effect of the β/β allele on weight gain induced by a high-fat diet (HF; D), and on monogenic obese mutants Cpefat/fat (E) and Lepob/ob (F). Each bar represents the mean ± S.E.M. (n = 5-8). (Std) standard diet; (*) p < 0.05; (**) p < 0.01 between the two groups of mice indicated.
We next introduced the β/β alleles into Lepob/ob and Cpefat/fat mice. Cpefat/fat mice do not consume more food than normal mice, but become obese due to an energy expenditure problem, whereas Lepob/ob mice are obese in part because of an excessive food intake (Zhang et al. 1994; Naggert et al. 1995). β/β × Lepob/ob and β/β × Cpefat/fat pups, carrying homozygous β/β alleles, appeared normal prior to weaning, but grew much slower than their respective littermates, Lepob/ob and Cpefat/fat (Fig. 1E,F). Although β/β × Cpefat/fat and Cpefat/fat mice had the same food intake, at 40 wk of age, the Cpefat/fat mice of both sexes were 120% heavier than their heterozygous littermates, but the β/β × Cpefat/fat mice were only 40% heavier (Fig. 1E). Similarly, weight gain in β/β × Lepob/ob mice was markedly reduced. At 40 wk of age, Lepob/ob mice of both sexes were >150% heavier than their heterozygous littermates, whereas the male and female β/β × Lepob/ob mice were only 85% and 45% heavier, respectively (Fig. 1F). The amount of food eaten did not differ significantly between male Lepob/ob and β/β × Lepob/ob mice, but female β/β × Lepob/ob mice consumed 50% less than female Lepob/ob mice. β/β × Lepob/ob mice, despite exhibiting a lower weight gain, showed a hyperinsulinemia, hyperglycemia, and infertility similar to their Lepob/ob littermates (data not shown), indicating that the β/β alleles were unable to improve these leptin-deficient physiological states. Nevertheless, β/β × Lepob/ob mice had increased longevity (23.1 ± 4.7 mo) compared with their Lepob/ob littermates (18 ± 4 mo; Fig. 1A). Taken together, homozygous β/β alleles efficiently prevented marked weight gain, but not the other abnormalities associated with the loss of Lep or Cpe gene function.
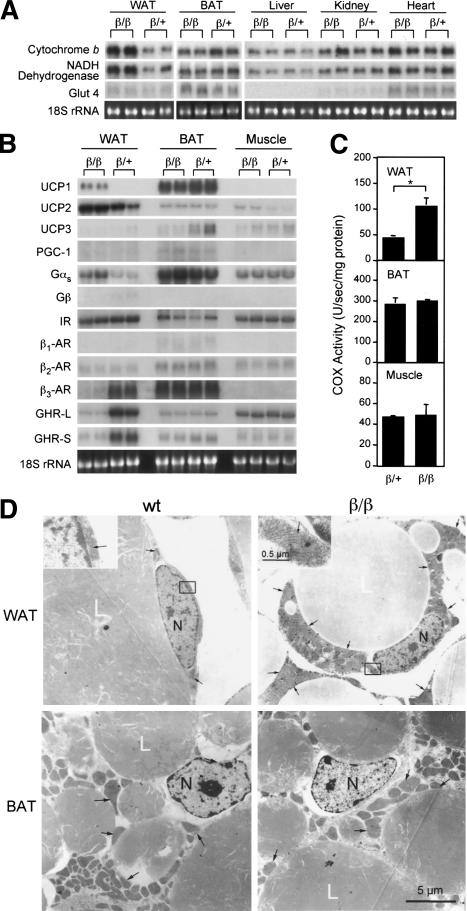
To assess the energy expenditure profile of β/β mice, we assayed various β/β mouse tissues for the expression of genes involved in mitochondrial oxidative respiration and their enzymatic activity (Scheffler 1999). The expression of genes encoding cytochrome b and NADH dehydrogenase and the activity of cytochrome c oxidase (COX) in WAT of β/β mice were markedly higher than that of their heterozygous littermates (Fig. 2A,C). WAT is designed for fat storage, whereas brown adipose tissue (BAT) is used for fat combustion. Surprisingly, WAT was the only tissue to possess elevated COX activities in β/β mice (Fig. 2; data not shown), whereas there was no difference in COX activity in the muscle and BAT, the two major sites for energy burning and thermogenesis (Lowell et al. 1993; Flier 1995), of β/β mice and their heterozygous littermates. Structurally, the mitochondrial content of WAT adipocytes is low, whereas BAT adipocytes are enriched with mitochondria (Nicholls and Loche 1984; Loncar et al. 1988). The high level of mitochondrial biogenesis in BAT is indicative of an extremely high metabolism. The increased energy oxidation in the WAT of β/β mice as compared with heterozygous mice is likely to have resulted from an increase in metabolically active mitochondria. Electron microscopy showed that the mitochondrial volume was dramatically increased in the epididymal WAT adipocytes of β/β mice as compared with that of their heterozygous littermates (Fig. 2D). The mitochondria in the WAT adipocytes of β/β mice are full of straight or slightly wavy cirstae that transverse the width of the mitochondria (Fig. 2D, top, left windows), thereby increasing their surface area—a typical characteristic of metabolically active mitochondria (Loncar et al. 1988). The BAT adipocytes of β/β mice in comparison to heterozygous mice, however, did not possess this increase in mitochondria or changes in mitochondrial activity (Fig. 2D). BAT utilizes UCPs to uncouple oxidative phosphorylation in the mitochondrial Electron Transfer Chain reaction, thereby reducing ATP production, and thus efficiently dissipating energy as heat (Nicholls and Loche 1984). UCPs are expressed in a tissue-specific manner; UCP1 is BAT specific, UCP2 is expressed in most tissues, whereas UCP3 is found in BAT and skeleton muscle (Jacobsson et al. 1985; Boss et al. 1997; Fleury et al. 1997). Interestingly, UCP1 mRNA levels were markedly elevated in WAT but not in other tissues of β/β mice (Fig. 2B), indicating that the WAT of β/β mice might have dissipated energy as heat in a similar manner to that of BAT. This would explain the higher body temperatures seen in β/β mice.
Figure 2.
Mitochondria-enriched WAT and increased expression of genes encoding factors involved in energy oxidation in WAT of β/β mice. Northern blot analyses of enzyme genes involved in mitochondrial Electron Transfer Chain (A) and regulators (B) in tissues of β/β mice. Each lane contains 10 μg RNA from an individual animal. (C) Cytochrome c oxidase (COX) activity of tissue extracts of β/β mice. Each bar represents the mean ± S.E.M. (n = 5). (*) p < 0.05 between the two groups of mice indicated. (D) Electron micrograph of adipocytes from epididylmal (WAT) and brown adipose tissues (BAT) of 4-week-old β/β mice. Top windows show a higher magnification of the rectangle-enclosed area on the WAT micrograph. (L) lipid droplets; (N) nucleus. Black arrows indicate representative mitochondria.
To understand the molecular mechanism by which β/β allele confers BAT-like characteristics to WAT of β/β mice, we used cDNA subtraction to analyze the WAT gene expression profile (Diatchenko et al. 1996). We found that the levels of mRNAs for the growth hormone receptor (GHR), both full-length (GHR-L) and spliced (GHR-S), and β3 adrenergic receptor (AR), were significantly reduced in WAT, whereas the mRNA levels for insulin receptor (IR) remained unaffected (Fig. 2B). This indicated that growth hormone and adrenergic factor signal transductions might be compromised in WAT of β/β mice. Interestingly, the expression of Gαs, which couples with growth factor receptors such as β-AR (Neves et al. 2002) to mediate their activation, was markedly increased in WAT of β/β mice (Fig. 2B). Also increased were the cellular levels of cAMP, an intracellular signal transducer for the activated Gαs, which was 65% higher in the WAT of β/β mice (18.1 ± 2.3 pmole/mg protein) than that of β/+ mice (11.7 ± 1.4 pmole/mg protein).
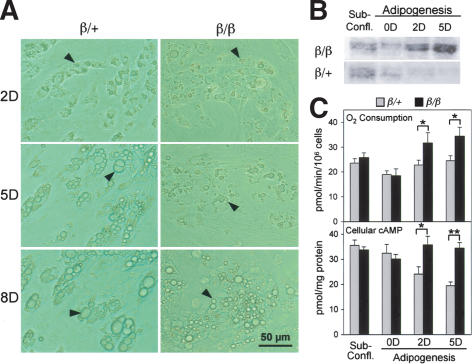
Markedly increased mitochondrial content, elevated oxidative activity, and altered gene expression were observed only in the WAT of β/β mice. To determine whether these changes were associated with the maturation status of adipocytes, we isolated preadipocytes directly from epididymal fat pads of the respective mice, and hormonally induced them to become lipid-rich cells that were subsequently sorted and collected for further analyses using flow cytometry (Lee et al. 2004). Small lipid droplets were detected microscopically 2 d after adipogenic induction in both β/+ and β/β cells, indicating that their speed of adipogenic conversion did not differ significantly (Fig. 3A). However, similar to the WAT of β/β mice, lipid accumulation in β/β cells afterward appeared slower than in β/+ cells, as measured by the size and number of lipid droplets (Fig. 3A). Oxygen consumption and Gαs protein levels were initially similar between β/+ and β/β cells, but were significantly higher in the lipid-rich β/β cells afterward (Fig. 3B,C). These results indicated that the elevated levels of Gαs and mitochondrial biogenesis found in the WAT of β/β mice might have occured after the conversion of preadipocytes to lipid-rich cells. The levels of cellular cAMP were also elevated in the lipid-rich β/β cells (Fig. 3C), which could be associated with the effect of the increased expression of Gαs on adenylyl cyclase (AC) catalyzing the formation of cAMP (Gilman 1987). Whereas the IR-, β-AR-, and GHR-mediated regulatory networks all play an important role in regulating lipid storage in fat cells by affecting their lipolysis and/or lipogenesis, the Gαs-coupled β-AR pathway has well-documented thermogenic effects through the action of UCP1 (Nam and Lobie 2000; Saltiel and Kahn 2001; Arch 2002; Lowell and Bachman 2003). To determine whether the elevated levels of Gαs and its associated cAMP are involved in preventing lipid accumulation in β/β cells, we used an inhibitor of AC, 9-cyclopentyl adenine (9-CP-Ade), to suppress the AC regulatory pathway in the lipid-rich β/β cells. In the presence of 9-CP-Ade, β/β cells were able to increase their lipid storage to a degree comparable to that seen in β/+ cells, as judged by the size and number of lipid droplets formed inside cells (data not shown). This indicated that the elevated expression of Gαs and its associated regulatory network were involved in suppressing lipid accumulation in β/β cells.
Figure 3.
The increased expression of Gαs protein during adipogenesis of cells derived from WAT of β/β mice. (A) Light micrograph of cells isolated from epididylmal fat pads of β/β mice after adipogenic induction. The numbers on the left of the micrograph indicate the days after adipogenic induction. Black arrows indicate representative lipid droplets. Cellular Gαs protein (B) and oxygen consumption and camp levels (C) of lipid-rich cells after adipogenic induction of the cells isolated from epididylmal fat pads of β/β mice. Each bar represents the mean ± S.E.M. (n = 4). (*) p < 0.05; (**) p < 0.01 between the two groups of mice indicated.
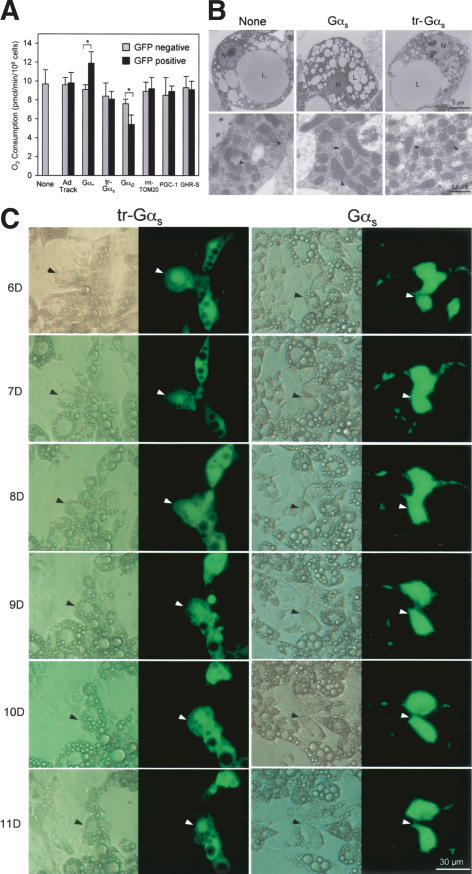
We next studied the direct effect of Gαs on lipid-rich cells. Various factors, including Gαs, were delivered into lipid-rich 3T3-L1 cells via a recombinant adenoviral vector (Ad) carrying a GFP expression cassette, allowing infected cells to be identified (He et al. 1998). The infected lipid-rich 3T3-L1 cells were separated from noninfected cells in the same culture using flow cytometry and analyzed with their electron micrography, O2 consumption, and lipid accumulation. The Ad.Gαs-infected cells showed higher O2 consumption than uninfected cells (Fig. 4A). In contrast, cells infected with Ad.Gαi2 had lower O2 consumption when compared with uninfected cells, whereas cells infected with Ad carrying only GFP (Ad.Track), PGC-1, GHR-S, mt-TOM20 (translocase of mitochondrial outer membrane, subunit 20), or a truncated form of Gαs lacking the N-terminal 36 amino acid residues required for membrane targeting and direct contact with Gβγ-dimer (Evanko et al. 2000; indicated as tr-Gαs) did not exhibit any change in O2 consumption (Fig. 4A). When examined by electron microscopy, only the cells infected with Ad.Gαs contained enlarged mitochondria full of cristae similar to those seen in the WAT of β/β mice, (Fig. 4B). Furthermore, upon light microscopic analysis of cellular lipid accumulation, cells infected with Ad.Gαs were found to lose their cellular lipid droplets by graduate shrinkage, whereas uninfected cells and those infected with Ad carrying other factors contained lipid droplets that were progressively enlarged and later merged together (Fig. 4C). Taken together, overexpression of Gαs, without addition of a β-AR agonist, effectively increased mitochondrial volume and O2 consumption, and subsequently reduced lipid accumulation in lipid-rich cells. This suggests that Gαs might play an active role, in addition to mediating the effect of β-AR activation, in programming the lipid-rich cells to be efficient in energy oxidation. Moreover, the truncated form of Gαs did not elicit the same effect as that of full-length Gαs, suggesting that the interaction with Gβγ-dimer is needed for mitochondrial biogenesis.
Figure 4.
Effects of Gαs on mitochondrial biogenesis and oxygen consumption in lipid-rich 3T3-L1 cells. (A) Oxygen consumption of lipid-rich 3T3-L1 cells infected with adenoviral vectors expressing the various factors indicated. Each bar represents the mean ± S.E.M. (n = 4). (*) p < 0.05 between the two groups of mice indicated. (B) Electron micrograph of lipid-rich 3T3-L1 cells infected with adenoviral vectors expressing Gαs protein. (Right) Higher magnifications of the rectangle-enclosed areas shown at left. (L) lipid droplets; (N) nucleus. Black arrowheads show representative mitochondria. (c) Light micrograph of lipid-rich 3T3-L1 cells infected with adenoviral vectors expressing Gαs proteins. Each micrograph shows both light (left) and dark (right, fluorescent) fields in parallel. Black and white arrowheads point to the same cell shown in light and dark fields, respectively. The numbers on the left of the micrograph indicate the number of days after adipogenic induction.
The GTPase cycle between Gβγ-dimer and Gα subunit in G protein-coupled receptor signaling is well established (Gilman 1987; Neves et al. 2002). However, a number of effectors have been shown to be regulated by Gβγ-dimer via direct protein interaction. These effectors include several AC isoforms, voltage-gated Ca2+ channels, and muscarinic K+ channels (Gautam et al. 1998). For example, Gβγ-dimer is a stimulator of Type II and Type IV AC isoforms, but an inhibitor of Type I AC (Simonds 1999). The Gβγ-dimer may also exert its cellular effect indirectly by interaction with other proteins, such as calmodulin and mitogen-activated protein kinase (Schwindinger and Robishaw 2001). In the WAT fat cells of β/β mice, it is unlikely that the effect of Gαs was in the main associated with the activation of β-ARs, because β-AR expression was markedly reduced in the WAT of β/β mice, possibly due to the lack of C/EBPα (Dixon et al. 2001). One possibility is that the elevated levels of Gαs effectively compete with other Gα subunits for the free form of Gβγ-dimer, and subsequently prevent the Gβγ-dimer and/or activation of other Gα subunits from exerting their cellular effects that may include inhibiting AC activity.
Adipocytes express all three subtypes of β-AR (β1, β2, and β3), of which each is coupled to the Gαs-cAMP pathway. Thus, in adipocytes, especially of BAT, Gαs has had its role in mediating signaling from the β-AR activation to elicit lipolysis and thermogenesis well documented (Collins and Surwit 2001; Kelly and Scarpulla 2004). An inhibitory role of Gαs in adipogenesis of 3T3-L1 cells has been suggested on the basis of the observations that Gαs expression declines markedly within 24 h of adipogenic induction, and that antisense Gαs oligodeoxynucleotides accelerate adipocyte differentiation (Wang et al. 1992). In postdifferentiation, however, the regulatory role of Gαs in adipocyte constitution is not yet defined. Interestingly, C/EBP gene replacement increased and maintained the Gαs expression specifically at the state of postadipogenic induction when the preadipocytes isolated from WAT of β/β mice were adipogenically induced. This was corroborated by the WAT-specific increase in Gαs expression in β/β mice. The increases in mitochondrial biogenesis and metabolic activity in lipid-rich 3T3-L1 cells after overexpression of Gαs at the postadipogenic induction stage further suggests an active role of Gαs in defining adipocyte composition during adipocyte differentiation.
In conclusion, this study indicates that the β/β allele changes the metabolic state of WAT adipocytes from energy storage to energy dissipation, possibly via an increased expression of Gαs. The increase in energy oxidation alone in fat cells appears to be able to reverse both genetic and dietary obesities. Regardless of the cause of obesity, all forms of obesity lead to an accumulation of massive quantities of fat in WAT. Thus, increasing the oxidative activity of WAT might be an effective treatment for obesity. Moreover, as overexpression alone of Gαs effectively increased mitochondrial biogenesis and prevented fat accumulation in lipid-rich cells, Gαs might play an active role in programming the lipid-rich cells to be as efficient energy oxidizers as the adipocytes of BAT in which Gαs is highly expressed.
Materials and methods
Mice
Cpefat/+ and Lepob/+ heterozygous mice were obtained from the Jackson Laboratory and bred with C/ebpαβ/β mice (Chen et al. 2000; here referred to as β/β mice, homozygous for a C/EBPβ gene knock-in at the C/ebpα locus; β/+ mice carry one C/ebpβ allele at the C/ebpα locus; both mice are wild type at the C/ebpβ locus) to generate the respective heterozygous double-mutant mice. These double-mutant heterozygous mice were then interbred to obtain homozygous double-mutant mice, β/β × Cpefat/fat and β/β × Lepob/ob, respectively.
High-fat diet experiments
Mice were kept in a sterile microisolator and were observed closely throughout the experiment. To measure the effect of HF diet, six mice of each sex from both β/+ and β/β genotypes were placed separately in a microisolator 2.5 wk after birth. Fresh food of standard (4% fat) or high fat (30% fat, LabDiet) was provided daily, and the amount of food consumed was recorded daily. To monitor growth rates, the body weight of mice was measured twice a week.
COX activity assay and cAMP measurement
The COX activity assay was used to assess mitochondrial respiratory activity (Cooperstein and Lazarow 1951). Frozen tissues were homogenized in 10 vol of ice-cold 30 mM phosphate buffer (pH 7.4). A total of 5-20 μL of tissue homogenate were then added to 0.5 mL of reduced cytochrome c solution. The reactions were measured at 550 nm in a spectrometer, with readings taken every 30 sec. Cellular cAMP contents were measured by a cAMP EIA kit (Biomedical Technologies).
Mouse respiration parameters
The respiration rate, O2 consumption, and CO2 production of individual mice, conscious but restrained, were measured using a respiration system coupled with O2/CO2 sensors (Kent Scientific, Inc). Each mouse was measured in the system at room temperature for 3 min, and twice a day for 3 consecutive days. The final value for each mouse was the average of six measurements.
Preadipocyte isolation and culture
Mouse preadipocytes were isolated from male epidedylmal fat pads and cultured accordingly (Zuk et al. 2001). Similar to 3T3-L1 cells, adipogenesis was induced by adding insulin, 3-isobutyl-1-methyl-xanthine (IBMX) and dexamethasone to 2-d postconfluent cells (designated Day 0) at final concentrations of 10 μg/mL, 0.5 mM, and 1 μM, respectively for 2 d (Sadowski 1992). In addition, 1 μM of BRL49653C (rosiglitazone, Smithkline Beecham) was included in the induction medium. Cells were then maintained in culture medium containing 2 μg/mL of insulin until needed.
Flow cytometry and cell sorting
Adipogenically induced cells were analyzed and sorted in a flow cytofluorometer (FACStar Plus, Becton Dickinson) using FACS (fluorescence-activated cell sorter) technology (Lee et al. 2004). Cells were dissociated with trypsin, washed twice with PBS, resuspended in cold PBS, and kept on ice before flow cytometric analysis. After sorting, the collected fat-laden cells were returned to culture medium and incubated at 37°C for at least 12 h before further analyses.
Recombinant adenovirus generation and infection
Recombinant viral vectors carrying cDNA encoding the indicated factors were made using the Ad Easy system (Quatum Biol. Inc). The plasmid pAdTrack CMV (He et al. 1998) containing a GFP marker gene and a CMV promoter for expression of the desired factor was used as the transfer vector for viral DNA homologous recombination in bacteria. After homologous recombination, adenoviral genomic DNA having a cDNA correctly integrated was transfected into 293 cells for virus production according to manufacturer's protocol. To infect the adipogenically induced 3T3-L1 cells, recombinant adenoviruses were added directly to the culture medium at the dosage of 50 m.o.i., and incubated for 24 h.
Cell O2 consumption
Immediately prior to measurement, cells were harvested by trypsinization, washed twice in PBS, and resuspended in 0.2 mL of PBS. Cells were then added to an Oxytherm oxygen electrode (HansaTech) pre-equilibrated and stabilized in 0.5 mL of oxygen buffer with air at 37°C. Oxygen consumption was monitored for 10 min, and the value was calculated according to the manufacturer's instruction. The electrode buffer contained 0.3 M mannitol, 10 mM KCl, 5 mM MgCl2, 10 mM KH2PO4, and 1 mg/mL BSA (pH 7.4).
Acknowledgments
We thank Dimitry Spitkovsky and Rudolf Wiesner for invaluable help with the generation of recombinant adenoviral vectors. This work was supported in part by the NSC grant 92-2311-B001-042 and the Alexander von Humboldt Foundation.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article published online ahead of print. Article and publication date are at http://www.genesdev.org/cgi/doi/10.1101/gad.1213104.
References
- Arch J.R.S. 2002. β3-adrenoceptor agonists: Potential, pitfalls and progress. Euro. J. Pharm. 440: 99-107. [DOI] [PubMed] [Google Scholar]
- Boss O., Samec, S., Paoloni-Giacobino, A., Rossier, C., Dulloo, A., Seydoux, J., Muzzin, P., and Giacobino, J.P. 1997. Uncoupling protein-3: A new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett. 408: 39-42. [DOI] [PubMed] [Google Scholar]
- Chen S.S., Chen, J.F., Johnson, P.F., Muppala, V., and Lee, Y.H. 2000. C/EBPβ, when expressed from the C/ebpα gene locus, can functionally replace C/EBPα in liver but not in adipose tissue. Mol. Cell Biol. 20: 7292-7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. and Surwit, R.S. 2001. The β-adrenergic receptors and the control of adipose tissue metabolism and thermogenesis. Recent Prog. Horm. Res. 56: 309-328. [DOI] [PubMed] [Google Scholar]
- Cooperstein S.J. and Lazarow, A. 1951. A microspectrophotometric method for the determination of cytochrome oxidase. J. Biol. Chem. 189: 665-670. [PubMed] [Google Scholar]
- Diatchenko L., Lau, Y.F.C., Campbell, A.P., Chenchik, A., Moqadam, F., Huang, B., Lukyanov, S., Lukyanov, K., Gurskaya, N., Sverdlov, E.D., et al. 1996. Suppression subtractive hybridization: A method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc. Natl. Acad. Sci. 93: 6025-6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon T.M., Daniel, K.W., Farmer, S.R., and Collins, S. 2001. CCAAT/enhancer-binding protein is required for transcription of the 3-adrenergic receptor gene during adipogenesis. J. Biol. Chem. 276: 722-728. [DOI] [PubMed] [Google Scholar]
- Evanko D.S., Thiyagarajan, M.M., and Wedegaertner, P.B. 2000. Interaction with Gβγ is required from membrane targeting and palmitoylation of Gαs and Gαq. J. Biol. Chem. 275: 1327-1336. [DOI] [PubMed] [Google Scholar]
- Fleury C., Neverova, M., Collins, S., Raimbault, S., Champigny, O., levi-Meyrueis, C., Bouillaud, F., Seldin, M.F., Surwit, R.S., Ricquier, D., et al. 1997. Uncoupling protein-2: A novel gene linked to obesity and hyperinsulinemia. Nat. Genet. 15: 269-272. [DOI] [PubMed] [Google Scholar]
- Flier J.S. 1995. The adipocyte: Storage depot or node on the energy information superhighway? Cell 80: 15-18. [DOI] [PubMed] [Google Scholar]
- ____. 2004. Obesity wars: Molecular progress confronts an expanding epidemic. Cell 116: 337-350. [DOI] [PubMed] [Google Scholar]
- Gautam N., Downes, G.B., Yan, K., and Kisselev, O. 1998. The G-protein βγ complex. Cell Signal 10: 447-455. [DOI] [PubMed] [Google Scholar]
- Gilman A.G. 1987. G proteins: Transducers of receptor-generated signals. Annu. Rev. Biochem. 56: 615-649. [DOI] [PubMed] [Google Scholar]
- He T.C., Zhou, S., da Costa, L.T., Yu, J., Kinzler K.W., and Vogelsterin, B. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. 95: 2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson A., Stakler, U., Glotzer, M.A., and Kozak, L.P. 1985. Mitochondrial uncoupling protein from mouse brown fat. Molecular cloning, genetic mapping, and mRNA expression. J. Biol. Chem. 260: 16250-16254. [PubMed] [Google Scholar]
- Kelly D.P. and Scarpulla, R.C. 2004. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes & Dev. 18: 357-368. [DOI] [PubMed] [Google Scholar]
- Kopelman P.G. 2000. Obesity as a medical problem. Nature 402: 635-643. [DOI] [PubMed] [Google Scholar]
- Lee Y.H., Chen, S.Y., Wiesner, R.J., and Huang, Y.F. 2004. Simple flow cytometric method used to assess lipid accumulation in fat cells. J. Lipid Res. 45: 1162-1167. [DOI] [PubMed] [Google Scholar]
- Loncar D., Afzelius, B.A., and Cannon, B. 1988. Epididymal white adipose tissue after cold stress in rats. II. Mitochondrial changes. J. Ultrastruct. Mol. Struct. Res. 101: 199-209. [DOI] [PubMed] [Google Scholar]
- Lowell B.B. and Bachman, E.S. 2003. β-adreergic receptors, diet-induced thermogenesis, and obesity. J. Biol. Chem. 278: 29385-29388. [DOI] [PubMed] [Google Scholar]
- Lowell B.B., S-Susulic, V., Hamann, A., Lawitts, J.A., Himms-Hagen, J., Boyer, B.B., Kozak, L.P., and Flier, J.S. 1993. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366: 740-742. [DOI] [PubMed] [Google Scholar]
- Moitra J.L., Mason, M.M., Olive, M., Krylor, D., Gavrilova, O., Marcus-Samuels, B., Feigenbaum, L., Lee, E., Aoyama, T., Eckhaus, M., et al. 1998. Life without white fat: A Transgenic mouse. Genes & Dev. 12: 3168-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naggert J.K., Fricker, L.D., Varlamov, O., Nishina, P.M., Rouille, Y., Steiner, D.F., Carroll, R.J., Paigen, B.J., and Leither, E.H. 1995. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat. Genet. 10: 135-142. [DOI] [PubMed] [Google Scholar]
- Nam S.Y. and Lobie, P.E. 2000. The mechanism of effect of growth hormone on preadipocte and adipocyte function. Obesity Rev. 1: 73-86. [DOI] [PubMed] [Google Scholar]
- Neves S.R., Ram, R.T., and Lyengar, R. 2002. G protein pathways. Science 296: 1636-1639. [DOI] [PubMed] [Google Scholar]
- Nicholls D.G. and Loche, R.M. 1984. Thermogenic mechanisms in brown fat. Physiol. Rev. 64: 1-64. [DOI] [PubMed] [Google Scholar]
- Rosen E.D., Sarraf, P., Troy, A.E., Bradwin, G., Moore, K., Milsotne, D.S., Spiegelman, B.M., and Mortensen, R.M. 1999. PPAR γ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4: 611-617. [DOI] [PubMed] [Google Scholar]
- Rosen E.D., Walkey, C.J., Puigserver, P., and Spiegelman, B.M. 2000. Transcriptional regulation of adipogenesis. Genes & Dev. 14: 1293-1307. [PubMed] [Google Scholar]
- Sadowski H.B., Wheeler, T.T., and Young, D.A. 1992. Gene expression during 3T3-L1 adipocyte differentiation. J. Biol. Chem. 266: 4722-4731. [PubMed] [Google Scholar]
- Saltiel A.R. and Kahn, C.R. 2001. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414: 799-806. [DOI] [PubMed] [Google Scholar]
- Scheffler I.E. 1999. Mitochondria, Wiley-Liss, Inc, New York.
- Schwindinger W.F. and Robishaw, J.D. 2001. Heterotrimeric G-protein βγ-dimers in growth and differentiation. Oncogene 20: 1653-1660. [DOI] [PubMed] [Google Scholar]
- Shimomura I., Hammer, R.E., Richardson, J.A., Ikemoto, S., Bashmakow, Y., Goldsten, J.L., and Brown, M.S. 1998. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: Model for congential generalized lipodystrophy. Genes & Dev. 12: 3182-3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds W.F. 1999. G protein regulation of adenylate cyclase. Trends Pharmacol. Sci. 20: 66-73. [DOI] [PubMed] [Google Scholar]
- Spiegelman B.M., Choy, L., Hotamisligil, G., Graves, R.A., and Tontonoz, P. 1993. Regulation of adipocyte gene expression in differentiation and syndromes of obesity/diabetes. J. Biol. Chem. 268: 6823-6826. [PubMed] [Google Scholar]
- Wang H.-Y., Watkins, D.C., and Malbon, C.C. 1992. Antisense oligodeoxynucleotides to Gs protein α-subunit sequence accelerate differentiation of fibroblasts to adipocytes. Nature 358: 334-337. [DOI] [PubMed] [Google Scholar]
- Wu Z., Bucher, N.L.R., and Farmer, S.R. 1996. Induction of peroxisome proliferator-activated receptor γ during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPβ, C/EBPδ, and glucocorticoids. Mol. Cell. Biol. 16: 4128-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh W.C., Cao, Z., Classon, M., and McKnight, S.L. 1995. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes & Dev. 9: 168-181. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Proenca, R., Maffei, M., Barone, M., Leopold, L., and Friedman, J.M. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425-432. [DOI] [PubMed] [Google Scholar]
- Zuk P.A., Zhu, M., Mizuno, H., Huang, J., Futrell, J.W., Katz, A.J., Benhaim, P., Lorenz, H.P., and Hedrick, M.H. 2001. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Engineering 7: 211-228. [DOI] [PubMed] [Google Scholar]