Abstract
Judgments of facial attractiveness are central to decision-making in various domains, but little is known about the extent to which they are malleable. In this study, we used EEG/ERP methods to examine two novel influences on neural and subjective responses to facial attractiveness: an observer’s expectation and repetition. In each trial of our task, participants viewed either an ordinary or attractive face. To alter expectations, the faces were preceded by a peer-rating that ostensibly reflected the overall attractiveness value assigned to that face by other individuals. To examine the impact of repetition, trials were presented twice throughout the experimental session. Results showed that participants’ expectations about a person’s attractiveness level powerfully altered both the neural response (i.e. the late positive potential; LPP) and self-reported attractiveness ratings. Intriguingly, repetition enhanced both the LPP and self-reported attractiveness as well. Exploratory analyses further suggested that both observer expectation and repetition modulated early neural responses (i.e. the early posterior negativity; EPN) elicited by facial attractiveness. Collectively, these results highlight novel influences on a core social judgment that underlies individuals’ affective lives.
Keywords: facial attractiveness, reappraisal, mere exposure effect, late positive potential, early posterior negativity, EEG/ERP
Introduction
We humans place considerable value on facial attractiveness in our lives as social animals. Judgments of others’ attractiveness guide decision-making in a broad range of domains, including mate and peer selection (Thornhill and Gangestad, 1999), professional choices (Landy and Sigall, 1974; Dipboye et al., 1977), and even beliefs about intellectual ability and moral character (Dion et al., 1972; Langlois et al., 2000). The perception of facial beauty also recruits key reward-related brain structures such as the nucleus accumbens and orbitofrontal cortex (Aharon et al., 2001; O’Doherty et al., 2003). Commensurate with its social importance, research suggests that the ability to evaluate facial attractiveness emerges early in life, within three days from birth (Slater et al., 1998, 2000).
To what extent are judgments of facial attractiveness malleable? A growing body of work suggests that facial beauty is determined by various objective characteristics of the stimulus. For instance, a face’s mathematical averageness (Langlois and Roggman, 1990), symmetry (Perrett et al., 1999; Penton-Voak et al., 2001), and the presence of sexual dimorphisms—such as large jaw structure in men or full lips in women (Perrett et al., 1998)—contribute to facial beauty. Despite this, evaluations of facial attractiveness could also be amenable to change. In this study, we examined two influences on neural (EEG/ERP) and subjective responses to facial attractiveness: an observer’s expectation about a person and repetition.
Facial attractiveness: the role of observer expectations
Individuals must use currently available information to predict and form responses to future events, and this ability to guide behavior according to expectations is crucial to adaptive functioning (Posner, 1980; Schultz, 2000). Expectations allow us to attribute value to specific events in an uncertain future, and thereby direct behavior and limited cognitive resources accordingly. Indeed, a variety of studies suggest that our expectations of to-be-encountered stimuli shape our response to them. For instance, information provided before the onset of painful stimulation (Koban and Wager, 2016), aversive scenes (Foti and Hajcak, 2008), or expressions of emotion (Wieser et al., 2014) reliably changes both self-reported and physiological responses to them. These studies highlight the possibility that judgments of facial attractiveness may also be sensitive to individuals’ expectations, but this hypothesis has not yet been directly examined.
Suggestive evidence indicates, however, that evaluations of facial beauty are sensitive to other cognitive factors. Morgan and Kisley (2014) recently showed that beliefs about one’s own mate value is linked to evaluations of others’ attractiveness. Little et al. (2006) provided evidence that individuals’ desired personality traits in a romantic partner impact their judgments of facial attractiveness. Furthermore, Bronstad and Russell (2007) showed that close relations (e.g. siblings, spouses, friends, etc.) had greater agreement on evaluations of facial attractiveness than strangers, attesting to its potential sensitivity to social networks. In an interesting recent twin-design study aimed at identifying the relative contributions of environmental vs genetic factors on judgments of facial beauty, Germine et al. (2015) found that individuals’ environments (and not genes) accounted for most of the variance in such appraisals. Collectively, these findings attest to the apparent cognitive malleability of facial beauty and raise the intriguing possibility that what we expect to see in people shapes our evaluations of their attractiveness.
Facial attractiveness: the role of repetition
A secondary question on the malleability of facial attractiveness involves the role of repetition. In our intricate social networks, we usually encounter individuals on more than a single occasion. Often, these encounters are situated within some context, such as prior beliefs held about the individual. At present, however, little is known about how repeated exposures to a person influence judgments of facial attractiveness. An extensive body of work on the mere exposure effect (Zajonc, 1968) suggests that repeated encounters with a stimulus enhance liking for it. This has been demonstrated with a variety of affectively neutral stimuli, including shapes, tones and ambiguous Chinese letters (Zajonc, 2001). Such effects are widely attributed to enhanced fluency of cognitive processing arising from mere repetition (Reber et al., 1998). Thus, the mere exposure effect would predict that the attractiveness of faces increases with repetition.
Intriguingly, a parallel line of research on affective adaptation suggests that the rewarding value of a stimulus could actually decrease with subsequent exposure (see Wilson and Gilbert, 2008 for a review). This line of work argues that attending to and evaluating a positive stimulus may reduce some of its hedonic qualities, dampening its reward value upon re-exposure. Thus, affective adaptation predicts that the attractiveness value of faces may diminish with repetition.
Methodological considerations
A number of studies have shown that attractive (vs ordinary) faces consistently elicit a larger late positive potential (LPP; Johnston and Oliver-Rodriguez, 1997; van Hooff et al., 2010; Morgan and Kisley, 2014), an EEG-based waveform that begins ∼400 ms after stimulus onset and is maximal at parietal areas of the scalp (see Hajcak et al., 2010 for a review). The LPP is sensitive to the affective significance of a stimulus (Thiruchselvam et al., 2011, 2012) but not basic perceptual features such as image size (De Cesarei and Codispoti, 2006) or figure-ground complexity (Bradley et al., 2007). Thus, it would serve as a useful measure to assess how a face’s attractiveness is shaped by the observer’s expectations and repetition.
Prior studies have also revealed earlier ERP components to be sensitive to facial attractiveness (Werheid et al., 2007; Schacht et al., 2008; Trujillo et al., 2014) and repetition (Schweinberger et al., 2004). In particular, a negative waveform occurring in the 200–400 ms time range at temporal-occipital sites on the scalp—corresponding to the early posterior negativity (EPN; Schupp et al., 2004, 2007) or the N250 (Schweinberger et al., 2004; Wiese et al., 2014)—has been linked to a rapid identification process which assigns motivational significance to stimuli. Although the LPP has been shown to be reliably altered by emotion regulation techniques such as the cognitive reappraisal of affective stimuli (Foti and Hajcak, 2008; Thiruchselvam et al., 2011), evidence for the impact of cognitive change on this earlier ERP component remains sparse. Thus, in addition to our focus on the LPP, we performed exploratory analyses to examine whether an observer’s expectations about facial attractiveness would alter the EPN/N250. Furthermore, we sought to replicate prior findings on the impact of repetition of faces on the EPN/N250 (Schweinberger et al., 2004; Tanaka et al., 2006).
The present study
In this study, participants viewed high-attractive and low-attractive faces and rated their attractiveness. To manipulate expectations, each face was preceded by a peer-rating that ostensibly reflected the overall attractiveness level of that face as determined by other individuals. For the high-attractive faces, peer-ratings were either artificially increased (high expectation condition) or decreased (low expectation condition) relative to the mean attractiveness value for that face as determined by an independent sample. Furthermore, each trial (including both the face image and the preceding peer-ratings) was presented twice through the experimental session. This allowed us to address two core questions:
How does information (i.e. peer-ratings) viewed before a face alter neural and subjective responses to attractiveness?
How does repetition (i.e. of faces accompanied by their peer-ratings) shape these responses?
To address both questions, we combined assessments of EEG/ERP responses (i.e. the LPP) with self-reported attractiveness. Crucially, using a neural measure concomitantly with self-report allowed us to examine whether our manipulations (i.e. altering expectations) produced changes that could not be easily ascribed to simple demand effects. Furthermore, as exploratory analyses, we examined how the EPN/N250 was modulated by each of our manipulations.
Methods
Participants
Twenty-two undergraduate students from Hamilton College participated in this study (mean age: 19.63; range: 18–21 years; s.d.: 0.84). All participants were heterosexual (11 males, 11 females) and had normal or corrected-to-normal vision. Moreover, we only examined unattached (i.e. single) individuals, since research shows that being in a committed relationship can dampen the response to attractive individuals (Maner et al., 2008). Three participants received course credit, and 19 received payment. One female participant was excluded from analyses due to prior knowledge about the study. One female subject was not included in EEG analyses due to highly noisy data (i.e. more than 60% unusable trials), and another female subject was excluded from self-report analyses due to technical issues that rendered ratings unavailable.
Materials
Image stimuli
Facial images displaying relatively neutral expressions were collected from free online sources. In total, 56 high-attractive and 56 low-attractive faces for each gender category were used in the experimental task. All images were gray-scaled and cropped to fit 288 × 360 pixel dimensions. To determine mean attractiveness levels for the selected faces, the faces were pre-rated on attractiveness (using a 1–9 scale, anchored from ‘not at all attractive’ to ‘extremely attractive’) through a separate study by an independent sample of undergraduate Hamilton College students (N = 27; 16 females, 11 males). Images were assigned to experimental conditions on the basis of these ratings.
For both the female and male image sets, images categorized into the low-attractive face condition (males: M = 2.85, s.d. = 0.65; females: M = 3.44, s.d. = 0.57) had significantly lower ratings than those categorized as high-attractive (males: M = 6.55, s.d. = 0.83; females: M = 6.99, s.d. = 0.55); images were generally categorized as high-attractive if their mean rating was above 5, as that was the midpoint on our 1–9 attractiveness rating scale. Within each high-attractive face category, images were randomly divided into two subsets, so that they could be assigned to high vs low expectation conditions (see Procedure later). Great care was taken to ensure that the high-attractive faces assigned to each subset were as closely matched as possible in attractiveness ratings: male faces (Set 1: M = 6.54, s.d. = 0.91; Set 2: M = 6.55, s.d. = 0.76), female faces (Set 1: M = 7.01, s.d. = 0.47; Set 2: M = 6.97, s.d. = 0.63). Pairwise comparisons between the two subsets within each gender category were non-significant (P > 0.75 for each t-test).
The stimuli were presented using E-prime 2 (Schneider et al., 2002) in a sound-attenuated EEG chamber. Viewing distance was held constant at ∼20 inches.
Procedure
Upon arrival, participants completed informed consent and a set of questionnaires assessing personality measures.1 Participants were told that the study examines judgments of facial attractiveness, and that they would be shown information about how their peers on campus had rated the faces in an earlier study. Participants then completed an experimental task that involved viewing opposite-sex faces and rating their attractiveness.
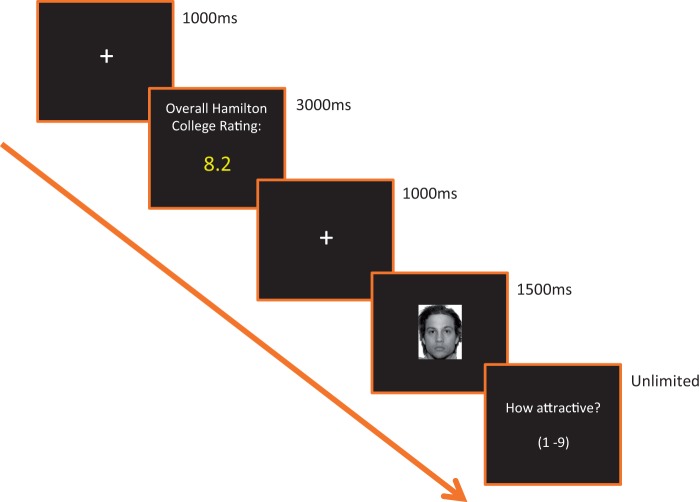
The trial structure is depicted in Figure 1. On each trial, subjects saw a fixation cross (1000 ms), a peer-rating ostensibly reflecting the overall attractiveness value assigned by campus students to an upcoming face (3000 ms), a second fixation (1000 ms), a face image (1500 ms), and a final rating screen to assess attractiveness on a 1–9 scale (unlimited duration). To assess the impact of repetition, the experimental session contained two separate phases, with each phase containing four experimental blocks.
Fig. 1.
Trial structure for the experimental task.
Each block of the first phase consisted of 28 trials: 14 low-attractive face trials and 14 high-attractive face trials. In the high-attractive face trials, seven were paired with a falsely inflated peer-rating (high-attractive face: high expectation), and seven were paired with a falsely lowered peer-rating (high-attractive face: low expectation). To manipulate peer-ratings in the high-attractive face: high expectation vs the high-attractive face: low expectation conditions, we added or subtracted 1.8 points from the face’s mean attractiveness value obtained from the independent sample.2 The peer-ratings shown in the low-attractive face condition reflected the actual mean attractiveness value obtained for that face from the independent sample. All trials were presented in a randomized order. The second phase of the experiment contained the same set of trials as the first phase, also presented in randomized order.
EEG recording, data reduction and analysis
Continuous EEG recordings were made from 32-electrodes using BrainVision’s actiCHamp (Brain Vision, Morrisville, NC). Cz served as the online reference. The EEG signal was recorded in DC mode and sampled at a rate of 500 Hz. Impedance levels were kept below 10 kΩ at all sites.
Offline, preprocessing was conducted using BrainVision’s Analyzer 2 software. To derive the LPP waveform, EEG data were filtered from 0.1 to 30 Hz and rereferenced to the average of the mastoids. Single-trial EEG epochs were extracted for a period beginning 200 ms before face image onset and continuing for the entire duration of image presentation (1500 ms). Epochs were baseline-corrected using the 200 ms prior to image onset. Trials were discarded due to excessive physiological noise if they contained: (i) an eye-blink, (ii) a voltage step > 50 µV/ms between sample points, (iii) a max–min difference > 150 µV/ms throughout the epoch, and (iv) low activity (i.e. <0.5 µV/ms) within a 100 ms window. This resulted in 89% of original trials remaining for analyses (90, 90 and 88% remaining in the high-attractive face: high expectation, high-attractive face: low expectation and low-attractive face conditions, respectively). On the basis of prior research (see Hajcak et al., 2010, for a review), we quantified the LPP as the average signal amplitude at site Pz in the 400–1200 ms time range after image onset.
To derive the EPN/N250 for our exploratory analyses, we applied the aforementioned processing stream, with two differences: (i) we used an average scalp reference in accordance with prior research (Schweinberger et al., 2004; Wiese et al., 2014)3 and (ii) single-trial EEG epochs were extracted for a period beginning 200 ms before face image onset until 600 ms. After artifact rejection on these epochs, ∼98% of trials remained for analyses in each of the three trial types. The EPN/N250 was quantified as the average activity at sites TP9/TP10, where visual inspection suggested the component was maximal, in the 200–400 ms time window.4
Results
In presenting our results, we first describe ANOVAs showing the main effects and interactions of our two manipulations (observer expectation and repetition) for each of our key dependent measures (LPP and self-reported attractiveness). To address the core questions of this study, we then proceed to unpack these ANOVAs with pairwise comparisons to examine the impact of each of our two manipulations on the LPP and self-reported attractiveness. Finally, we report exploratory analyses examining how our two manipulations each alter the EPN/N250 ERP component.
We first submitted participants’ LPP amplitudes to a repeated-measures ANOVA, with trial type (high-attractive face: high expectation, high-attractive face: low expectation, low-attractive face) and repetition level (first presentation, second presentation) as within-subjects factors. The ANOVA revealed a main effect of trial type [F(2,38) = 29.06, P < 0.001, p2 = 0.61], a main effect of repetition [F(1,19) = 6.24, P = 0.02, p2 = 0.25] and no interaction between trial type and repetition [F(2,38) = 0.49, ns].
We then analyzed participants’ self-reported attractiveness ratings. That ANOVA produced a main effect of trial type [F(2,38) = 328.49, P < 0.001,p2 = 0.95], a marginally significant effect of repetition level [F(1,19) = 3.33, P = 0.08, p2 = 0.15] and a marginally significant interaction between trial type and repetition level [F(2,38) = 2.42, P = 0.10, p2 = 0.11]. Later, we unpack these effects to address our two core questions about the impact of an observer’s expectation and repetition on responses to facial attractiveness.
Question 1: how do expectations alter judgments of facial attractiveness?
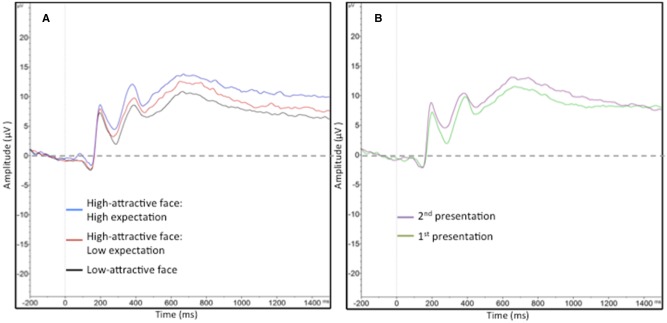
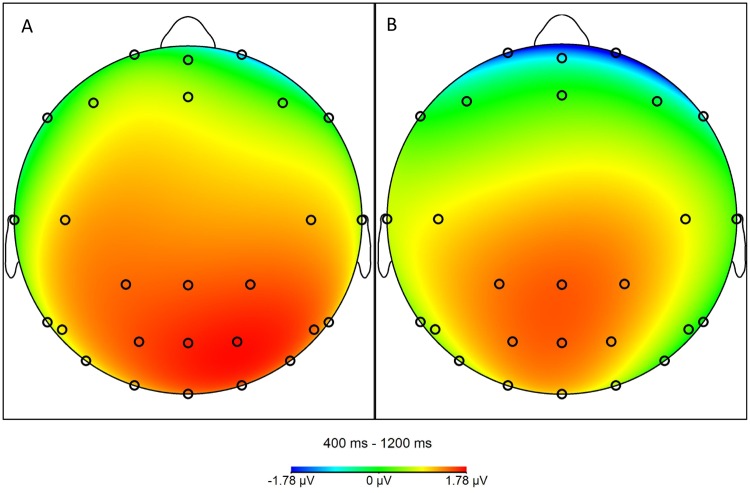
Planned pairwise comparisons on LPP amplitudes showed that both high-attractive face conditions (high-attractive face: high expectation: M = 11.39, s.d. = 4.51; high-attractive face: low expectation: M = 9.90, s.d. = 4.97) produced larger LPP responses than the low-attractive face condition (M = 8.08, s.d. = 4.36), (both P < 0.001). Critically, high-attractive face: high expectation had a larger LPP than the high-attractive face: low expectation condition, [t(19) = 3.58, P = 0.002]. The LPP for each trial type is presented in Figure 2, and the scalp voltage distribution of the difference between the two expectation conditions is shown in Figure 3.
Fig. 2.
LPP responses during face presentation by trial type (panel A) and repetition level (panel B).
Fig. 3.
Scalp voltage distribution contrasting high-attractive face: high expectation vs high-attractive face: low expectation (panel A) and first vs second presentation (panel B).
Mirroring the pattern obtained with the LPP, pairwise comparisons on self-reported attractiveness ratings showed that, as predicted, both high-attractive face conditions (high-attractive face: high expectation: M = 7.17, s.d. = 0.69; high-attractive face: low expectation: M = 6.68, s.d. = 0.70) produced higher ratings than the low-attractive face condition (M = 2.99, s.d. = 0.62), (both P < 0.001). The high-attractive face: high expectation condition produced greater ratings than the high-attractive face: low expectation condition [t(19) = 6.56, P < 0.001]. These are shown in Figure 4.
Fig. 4.
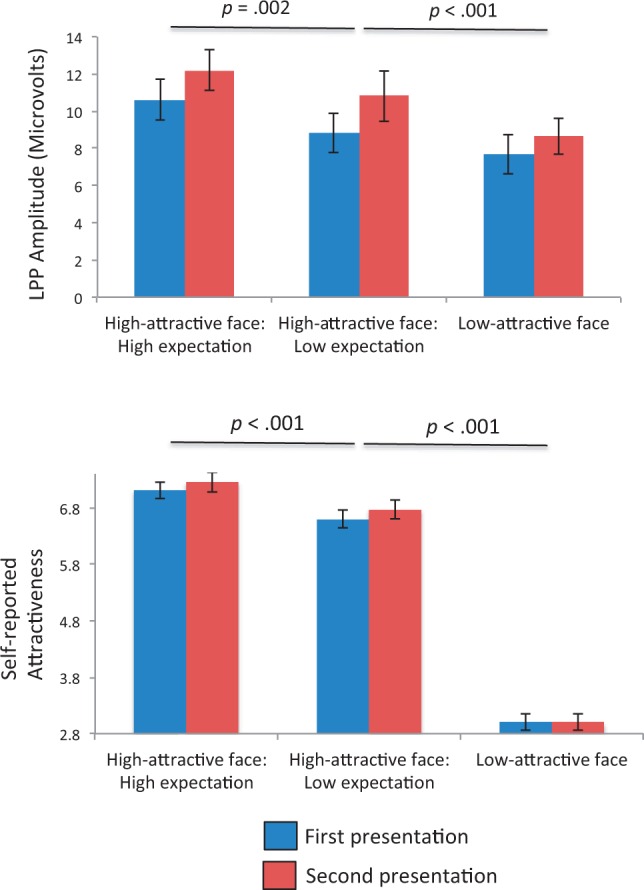
LPP amplitudes (top) and self-reported attractiveness (bottom) for the first and second presentation of each trial. Error bars reflect SEM. The P-values for pairwise comparisons between trial types are shown above the relevant bars.
Question 2: how does repetition influence judgments of facial attractiveness?
We then analyzed the LPP by repetition level (first vs second presentation) to examine the impact of re-exposure on responses to facial attractiveness. A comparison of LPP amplitudes in the first (M = 9.03, s.d. = 4.62) vs second (M = 10.55, s.d. = 4.77) presentation revealed that participants had higher LPPs upon the second presentation of each face condition, [t(19) = 2.49, P = 0.02].
As our ANOVA on self-reported attractiveness ratings produced a marginally significant interaction between trial type and repetition, we analyzed changes in ratings due to repetition for each trial type separately. For trials in the high-attractive face: high expectation condition, ratings were higher on the second presentation (M = 7.24, s.d. = 0.78) compared with the first (M = 7.10, s.d. = 0.63), [t(19) = 2.10, P < 0.05]. Similarly, for trials in the high-attractive face: low expectation condition, ratings were also greater on the second presentation (M = 6.76, s.d. = 0.72) relative to the first (M = 6.59, s.d. = 0.72), [t(19) = 2.01, P = 0.05]. For trials in the low-attractive face condition, however, there was no significant difference between the second (M = 2.99, s.d. = 0.65) vs first (M = 2.99, s.d. = 0.64) presentations (t < 1). In general, the non-parametric correlations (Spearman’s rho) for attractiveness ratings between the first and second presentation for each trial type were all highly significant (all P < 0.001).5 Changes in LPP amplitudes and self-reported attractiveness from first to second presentation are shown in Figure 4.
Exploratory analyses: EPN/N250
We then examined whether observer expectation and repetition impacted earlier ERPs, specifically the EPN/N250. We performed a repeated-measures ANOVA on mean EPN/N250 amplitudes with trial type (high-attractive face: high expectation, high-attractive face: low expectation, low-attractive face) and repetition level (first presentation, second presentation) as within-subjects factors. This revealed a main effect of trial type [F(2,38) = 12.16, P < 0.001, p2 = 0.39], a main effect of repetition level [F(1,19) = 9.61, P < 0.01, p2 = 0.34] and no interaction [F(2,38) = 0.72, ns].
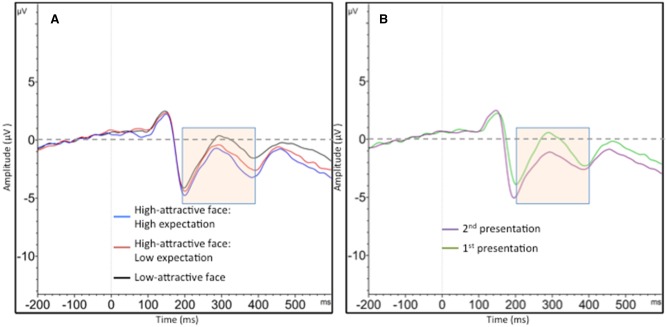
Follow-up t-tests suggested that the high-attractive face: high expectation condition (M = −2.27, s.d. = 2.76) produced a larger EPN/N250 than the high-attractive face: low expectation condition (M = −1.80, s.d. = 2.87), [t(19) = 2.23, P = 0.03]. Both high-attractive face conditions elicited a larger EPN/N250 than the low-attractive face type (M = −1.07, s.d. = 2.38), (both P < 0.02). With respect to the impact of repetition, the EPN/N250 was higher in the second presentation (M = −2.26, s.d. = 2.70) of the faces compared with the first (M = −1.17, s.d. = 2.72), [t(19) = 3.10, P < 0.01]. Figure 5 displays the EPN/N250 by trial type and repetition level.
Fig. 5.
EPN/N250 responses during face presentation by trial type (panel A) and repetition level (panel B). The time window (200–400 ms) in which the EPN was coded is highlighted for clarity. The waveforms reflect averaged activity between sites TP9 and TP10.
Discussion
In this study, we examined how an observer’s expectation and repetition altered neural and subjective responses to facial attractiveness. The findings showed that information about a person seen before a face powerfully influenced neural responses to their attractiveness, as evidenced by a modulated LPP. This was accompanied by robust changes in participants’ self-reported attractiveness of the faces. Furthermore, expectations modulated early neural responses—specifically, the EPN/N250—reflecting the rapid assignment of motivational significance to affective stimuli. These findings suggest that what we expect to see in a person can reliably influence both early and later neural responses, as well as subjective appraisals, of facial attractiveness. To the best of our knowledge, this is the first study to show that expectations (and more generally, beliefs about a person) can alter early and later EEG-derived neural responses elicited by facial attractiveness.
Second, the results showed that affective responses to facial attractiveness increase with repetition: both early and later ERP responses (the EPN/N250 and LPP) known to reflect affective reactivity, as well as the self-reported attractiveness of faces, were generally higher upon the second (vs first) presentation of each trial. Our findings are consistent with models of the mere exposure effect, proposing that repetition can increase the affective value of stimuli (Zajonc, 2001). A rich body of work has documented the mere exposure effect on a range of stimuli using self-reported liking (Bornstein, 1989; Monahan et al., 2000). Our findings add to that literature by showing that such effects are accompanied by specific neural changes that reflect affective response.
Implications for the cognitive change of facial attractiveness
Our pattern of findings highlights the malleability of responses to facial attractiveness, as both early and later ERP signals were effectively modulated by expectations held by the observer. The later LPP effects are consistent with a change in arousal elicited by facial beauty, since the LPP appears to track measures of autonomic activation (Cuthbert et al., 2000). This finding is also largely consistent with work in emotion regulation that shows successful modulation of the LPP with various cognitive strategies, including both the up-regulation and down-regulation of emotion using reappraisal (Hajcak et al., 2010; Gross, 2015).
The effect of peer-ratings on the earlier EPN/N250 is intriguing, as it suggests that top–down influences may exert a relatively deep impact in the evaluation of facial attractiveness. As the EPN/N250 is thought to reflect rapid attention toward events carrying basic motivational relevance, the reported effects suggest that an observer’s expectations could have altered (if only temporarily) the motivational significance assigned to a person’s facial beauty. It is also possible that the downstream effects observed in the LPP arose, at least in part, from cognitive changes that occurred during this early EPN/N250 stage.
It is important to note that, since self-reported responses are generally susceptible to demand effects (Nielsen and Kaszniak, 2007), such influences may have contributed to the observed impact of peer-ratings on self-reported attractiveness. However, one of the strengths of this study is that we assessed neural measures of affective response (LPP and EPN/N250) concurrently with self-report. Since the observed neural changes are unlikely to arise from demand effects, and as these converged closely with the pattern exhibited by the self-report data, it seems reasonable to conclude that expectations modulated affective responses from facial beauty.
A rich body of work—particularly from evolutionary psychology—suggests that facial beauty is determined by objective characteristics inherent to the stimulus, such as sexual dimorphisms, mathematical averageness, and symmetry (see Thornhill and Gangestad, 1999 for a review). From a functionalist perspective, such facial features are widely proposed to be attractive because they have served to signal reproductive fitness throughout evolutionary history, allowing individuals to assess mate value (Symons, 1979). This study suggests that responses to attractiveness may also be mediated through socially derived expectations. Indeed, prior work suggests that social information can affect basic sensory processes in face perception. Anderson et al. (2011) recently showed that faces paired with negative social information (e.g. gossip) persisted longer in visual consciousness in a binocular rivalry paradigm, compared with those paired with positive or neutral information. Individuals’ sensitivity to social information may be an especially potent influence on appraisals of the world, exerting a strong top–down impact on perceptual responses.
Implications for repeated exposure
Our findings suggest that affective responses to attractive faces become more pronounced upon re-exposure: the EPN/N250 and the LPP, as well as subjective attractiveness ratings, were generally heightened upon the second presentation of each face (relative to the first). Collectively, this finding offers one piece to resolve a puzzling phenomenon in human courtship: much to their surprise, people often find themselves drawn toward individuals after multiple encounters, even when there was no initial attraction. That is, cupid’s arrow is often slow to strike. Although multiple psychological changes are sure to contribute to this steady growth, an important part of the phenomenon may be attributable to the gradual change in attractiveness from repetition.
It is important to note that prior research on recognition memory has documented an ‘old/new effect’ whereby the LPP at parietal sites is heightened for remembered vs novel stimuli (see Rugg and Curran, 2007, for a review). Such ERP changes are often interpreted in non-affective terms, however, and attributed to strictly cognitive operations such as attentional orienting toward stored representations and graded recollection (Wilding, 2000; Rugg and Henson, 2002). Meanwhile, a robust literature in affective neuroscience links the parietal LPP to emotional arousal from affective stimuli (see Hajcak et al., 2010 for a review). These two literatures—the old/new phenomenon in memory research vs the LPP-arousal effect in emotion research—have typically proceeded in isolation. However, the present research opens up the intriguing possibility that the old/new LPP effect previously seen in memory research arises, at least in part, from the affective consequences of recollection. It is possible that successful recollection of memory items is accompanied by specific affective changes (i.e. familiarity or recognition itself might be rewarding, thereby enhancing the LPP). This possibility awaits further careful examination, which can be achieved by concurrently assessing both recollection accuracy and specific affective changes (e.g. facial EMG activity, alongside the LPP) that track it.
In a similar vein, prior research has shown the EPN/N250 to be enhanced both by facial attractiveness (Werheid et al., 2007; Wiese et al., 2014) as well as the repetition of face stimuli (Schweinberger et al., 2004; Tanaka et al., 2006). Our own results successfully replicate both of these independent findings. It is noteworthy that the EPN is widely attributed to an early operation of assigning attention toward events carrying basic affective significance (Schupp et al., 2007), whereas the N250 is studied in the context of memory processes often divorced from motivational considerations. However, the fact that both the EPN and N250 components show considerable spatial and temporal overlap highlights the possibility, as noted in the case of the old/new parietal LPP effect, that successful face recognition may be accompanied by certain affective consequences. Such face memory-affect links might precede the parietal LPP ‘old/new’ effect and be evident earlier during the EPN/N250 phase, but this also awaits further research that links memory performance and ERPs to measures that uniquely track affective response (e.g. facial EMG activity). It is worth noting, however, that unlike the parietal LPP ‘old/new’ effect that is observed with a range of stimulus types (e.g. words, pictures, etc.), the N250 repetition effect seems unique to face stimuli (Schweinberger et al., 2004).
The increase in self-reported attractiveness with repetition observed in this study aligns partly with a recent finding by Little et al. (2014), which showed that subjective ratings of beauty increase with exposure in women. Intriguingly, however, that study found ratings actually decrease with repetition in men. Working from an evolutionary framework, the authors interpreted their findings in terms of a heightened preference for sexual familiarity in women but sexual novelty in men. A potential explanation for the divergence between our own and Little et al.’s (2014) results among male subjects might arise from the relationship status of these participants.6 Little et al. (2014) did not appear to restrict their male participants to unattached (i.e. single) individuals, whereas we did. It is plausible that in males who are currently attached (vs single), and thus are not free to seek new mates, the tendency to find novel women arousing might be greater; in unattached males, however, this tendency might be relatively mute. Future studies can assess the role of relationship status in males’ (and females’) changes in perceptions of attractiveness with repeated exposure to stimuli. If evolutionary pressures differentially shaped males’ vs females’ preference for novelty, then the extent to which one is currently at liberty to seek novel mates should be expected to moderate this effect.
Limitations and future directions
It is important to note some limitations of this study that may be remedied by future research. First, we did not manipulate expectations in the low-attractive faces, which would have enabled us to examine the separate contributions of expectations vs face type on responses to attractiveness. This decision was partly necessitated by the fact that generating peer-ratings to decrease expectations in the low-attractive faces (i.e. by subtracting 1.8 points from the mean attractiveness value for each face derived from our independent sample) would lead a substantial number of faces in that condition to be assigned a peer-rating value of 1 (the lowest value on our scale). As this likely would have raised suspicion about the authenticity of the peer-ratings, we did not include such a manipulation. Future research, however, can resolve this by using faces of moderate (instead of low) attractiveness that enable the researcher to generate sufficiently low peer-ratings for each face without compromising the believability of the manipulation.
Similarly, we did not include a high-attractive face condition with unbiased peer-ratings to serve as a baseline against which to assess the impact of increasing vs decreasing expectations. This was done largely to preserve enough trials to elicit sufficiently strong signal-to-noise for detecting an effect of our manipulations, while keeping the experiment to a manageable length (i.e. to avoid subject fatigue). An interesting question for future research is whether people tend to weigh negative vs positive social information differently in appraisals of facial beauty. In a recent fMRI study, Korn et al. (2014) offered evidence that, across cultures, evaluations of character traits are more amenable to positive (vs negative) social information. Such findings support the possibility that positive vs negative social information is accorded varying levels of significance in shaping one’s judgments of attractiveness.
In addition, we restricted our stimuli to opposite-sex faces. Research suggests that individuals’ cognitive (e.g. attentional) and neural responses to opposite-sex vs same-sex faces differ (Johnston and Oliver-Rodriguez, 1997; Duncan et al., 2007). We expected that heterosexual individuals would show the most robust response to opposite-sex attractive faces, thereby providing a sufficiently strong signal for our manipulation (e.g. altering expectations) to exert its impact. Future research can examine the extent to which the effects reported in this study generalize across different stimulus categories (e.g. same-sex faces).
We found that repetition increased the LPP and EPN/N250 responses uniformly across all face types (high- and low-attractive faces) but that it increased self-reported attractiveness ratings only for high-attractive faces (i.e. ratings for low-attractive faces were unaffected by repetition). However, some caution is justified in interpreting this apparent divergence between the neural and self-report data; for the self-report ratings, the interaction in our ANOVA was marginally significant (although follow-up pairwise comparisons between repetition levels within each trial type was consistent with a pattern of interaction). Although the pattern of findings obtained with the neural and self-report measures appears largely consistent, it is unclear why repetition seems to have had no impact on self-report ratings for low-attractive faces. It is possible that these faces were actually somewhat negative (rather than neutral) in valence. As the mere exposure effect has been primarily observed in neutral and somewhat pleasant stimuli (Zajonc, 1968; 2001), faces of negative valence might not be as amenable to that effect. Indeed, recent studies that have examined mere exposure for unpleasant stimuli in other domains (e.g. odors) have failed to find an increase in self-reported liking, although neutral and mildly positive odors benefit from repetition (Delplanque et al., 2015).
Furthermore, our repetition manipulation involved presentations of both the face picture and the peer-ratings. We believe that this resembles real-life scenarios where encounters with an individual are often situated within some cognitive context, especially prior beliefs held about the person. However, this made it difficult to examine whether the peer-ratings vs the picture made separable contributions to the observed neural and self-report changes. Future studies can aim to disentangle these two components by independently manipulating the repetition of information about a person vs the picture itself.
One especially noteworthy future direction is to examine a dose–response relationship between repetition and neural responses of affect. Doing so would allow researchers to ask whether repetition influences affective responses beyond any contributions from recognition memory. More concretely, researchers can strive to hold memory performance constant (e.g. through ensuring near 100% memory accuracy by using a very small face stimulus set), and then examine whether the LPP and EPN/N250 increase as a function of repetition dose. As noted earlier, one possibility is that the enhanced LPP and EPN/N250 observed in this study actually arose, at least in part, from the affective consequences of successful recognition. However, if repetition contributes to affective responses beyond memory processes, then that should be evident in such an investigation.
Acknowledgements
The authors thank three anonymous reviewers for helpful suggestions with earlier drafts of the article and Emily Morris for assistance with stimulus collection.
Conflict of interest. None declared.
Footnotes
Data from these questionnaires are not presented because they are not central to the study’s main goals.
For nine of the high-attractive faces in each gender category, adding 1.8 points to the face’s mean attractiveness score produced a value > 9 (the upper limit on our rating scale). In these cases, we assigned those faces a maximum peer-rating value of 9.
We also confirmed that the reported EPN/N250 effects were present using a Cz reference, since the number of electrodes measured (32 sites) is less than that recommended for an average reference scheme (Junghöfer et al., 1999). The reported pattern of findings was indeed observed when using the Cz reference as well. See Trujillo et al. (2014) for a similar procedure.
Visual inspection of our data at occipitotemporal sites (i.e. TP9/TP10) suggested that facial attractiveness modulated the negative waveform beginning at 200 ms. Consistent with prior research (Wiese et al., 2014), we coded the EPN/N250 activity until 400 ms. The reported effects are still present when using the 270–400 ms window as employed by Wiese et al. (2014).
The r-values were 0.87, 0.80 and 0.88 in the high-attractive face: high expectation, high-attractive face: low expectation and low-attractive face conditions, respectively.
As an exploratory analysis, we examined in our data whether there were gender differences in the impact of repetition. A comparison of the difference score (second presentation—first presentation, merging across trial types) in our three key measures (LPP; EPN/N250; self-report) suggested that males and females did not differ significantly in the amount of change due to repetition (all t-tests produced P > 0.45). However, since our sample size for gender comparisons is small relative to that of Little et al. (2014), the lack of a gender difference should be interpreted with some caution.
References
- Aharon I., Etcoff N., Ariely D., Chabris C.F., O’Connor E., Breiter H.C. (2001). Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron, 32(3),537–51. [DOI] [PubMed] [Google Scholar]
- Anderson E., Siegel E.H., Bliss-Moreau E., Barrett L.F. (2011). The visual impact of gossip. Science, 332(6036),1446–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein R.F. (1989). Exposure and affect: overview and meta-analysis of research, 1968–1987. Psychological Bulletin, 106(2),265. [Google Scholar]
- Bradley M.M., Hamby S., Löw A., Lang P.J. (2007). Brain potentials in perception: picture complexity and emotional arousal. Psychophysiology, 44(3),364–73. [DOI] [PubMed] [Google Scholar]
- Bronstad P.M., Russell R. (2007). Beauty is in the ‘we’ of the beholder: greater agreement on facial attractiveness among close relations. Perception, 36(11),1674–81. [DOI] [PubMed] [Google Scholar]
- Cuthbert B.N., Schupp H.T., Bradley M.M., Birbaumer N., Lang P.J. (2000). Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology, 52(2),95–111. [DOI] [PubMed] [Google Scholar]
- De Cesarei A., Codispoti M. (2006). When does size not matter? Effects of stimulus size on affective modulation. Psychophysiology, 43(2),207–15. [DOI] [PubMed] [Google Scholar]
- Delplanque S., Coppin G., Bloesch L., Cayeux I., Sander D. (2015). The mere exposure effect depends on an odor’s initial pleasantness. Frontiers in Psychology, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion K., Berscheid E., Walster E. (1972). What is beautiful is good. Journal of Personality and Social Psychology, 24(3),285.. [DOI] [PubMed] [Google Scholar]
- Dipboye R.L., Arvey R.D., Terpstra D.E. (1977). Sex and physical attractiveness of raters and applicants as determinants of resumé evaluations. Journal of Applied Psychology, 62(3),288. [Google Scholar]
- Duncan L.A., Park J.H., Faulkner J., Schaller M., Neuberg S.L., Kenrick D.T. (2007). Adaptive allocation of attention: effects of sex and sociosexuality on visual attention to attractive opposite-sex faces. Evolution and Human Behavior, 28(5),359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D., Hajcak G. (2008). Deconstructing reappraisal: descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience, 20(6),977–88. [DOI] [PubMed] [Google Scholar]
- Germine L., Russell R., Bronstad P.M., et al. (2015). Individual aesthetic preferences for faces are shaped mostly by environments, not genes. Current Biology, 25(20),2684–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.J. (2015). Emotion regulation: current status and future prospects. Psychological Inquiry, 26(1),1–26. [Google Scholar]
- Hajcak G., MacNamara A., Olvet D.M. (2010). Event-related potentials, emotion, and emotion regulation: an integrative review. Developmental Neuropsychology, 35(2),129–55. [DOI] [PubMed] [Google Scholar]
- Johnston V.S., Oliver-Rodriguez J.C. (1997). Facial beauty and the late positive component of event-related potentials. Journal of Sex Research, 34, 188–98. [Google Scholar]
- Junghöfer M., Elbert T., Tucker D.M., Braun C. (1999). The polar average reference effect: a bias in estimating the head surface integral in EEG recording. Clinical Neurophysiology, 110(6),1149–55. [DOI] [PubMed] [Google Scholar]
- Koban L., Wager T.D. (2016). Beyond conformity: social influences on pain reports and physiology. Emotion, 16(1),24.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn C.W., Fan Y., Zhang K., Wang C., Han S., Heekeren H.R. (2014). Cultural influences on social feedback processing of character traits. Frontiers in Human Neuroscience, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy D., Sigall H. (1974). Beauty is talent: task evaluation as a function of the performer’s physical attractiveness. Journal of Personality and Social Psychology, 29(3),299. [Google Scholar]
- Langlois J.H., Kalakanis L., Rubenstein A.J., Larson A., Hallam M., Smoot M. (2000). Maxims or myths of beauty? A meta-analytic and theoretical review. Psychological Bulletin, 126(3),390.. [DOI] [PubMed] [Google Scholar]
- Langlois J.H., Roggman L.A. (1990). Attractive faces are only average. Psychological Science, 1(2),115–21. [Google Scholar]
- Little A.C., Burt D.M., Perrett D.I. (2006). What is good is beautiful: face preference reflects desired personality. Personality and Individual Differences, 41(6),1107–18. [Google Scholar]
- Little A.C., DeBruine L.M., Jones B.C. (2014). Sex differences in attraction to familiar and unfamiliar opposite-sex faces: men prefer novelty and women prefer familiarity. Archives of Sexual Behavior, 43(5),973–81. [DOI] [PubMed] [Google Scholar]
- Maner J.K., Rouby D.A., Gonzaga G.C. (2008). Automatic inattention to attractive alternatives: the evolved psychology of relationship maintenance. Evolution and Human Behavior, 29(5),343–9. [Google Scholar]
- Monahan J.L., Murphy S.T., Zajonc R.B. (2000). Subliminal mere exposure: specific, general, and diffuse effects. Psychological Science, 11(6),462–6. [DOI] [PubMed] [Google Scholar]
- Morgan L.K., Kisley M.A. (2014). The effects of facial attractiveness and perceiver’s mate value on adaptive allocation of central processing resources. Evolution and Human Behavior, 35(2),96–102. [Google Scholar]
- Nielsen L., Kaszniak A.W. (2007). Conceptual, theoretical, and methodological issues in inferring subjective emotional experience: Recommendations for researchers. In: Allen J.J.B., Coan J., editors. The Handbook of Emotion Elicitation and Assessment, (pp. 361–375), New York: Oxford University Press. [Google Scholar]
- O’Doherty J., Winston J., Critchley H., Perrett D., Burt D.M., Dolan R.J. (2003). Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia, 41(2),147–55. [DOI] [PubMed] [Google Scholar]
- Penton-Voak I.S., Jones B.C., Little A.C., et al. (2001). Symmetry, sexual dimorphism in facial proportions and male facial attractiveness. Proceedings of the Royal Society of London B: Biological Sciences, 268(1476),1617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett D.I., Burt D.M., Penton-Voak I.S., Lee K.J., Rowland D.A., Edwards R. (1999). Symmetry and human facial attractiveness. Evolution and Human Behavior, 20(5),295–307. [Google Scholar]
- Perrett D.I., Lee K.J., Penton-Voak I., et al. (1998). Effects of sexual dimorphism on facial attractiveness. Nature, 394(6696),884–7. [DOI] [PubMed] [Google Scholar]
- Posner M.I. (1980). Orienting of attention. Quarterly Journal of Experimental Psychology, 32(1),3–25. [DOI] [PubMed] [Google Scholar]
- Reber R., Winkielman P., Schwarz N. (1998). Effects of perceptual fluency on affective judgments. Psychological Science, 9(1),45–8. [Google Scholar]
- Rugg M.D., Curran T. (2007). Event-related potentials and recognition memory. Trends in Cognitive Sciences, 11(6),251–7. [DOI] [PubMed] [Google Scholar]
- Rugg M.D., Henson R.N.A. (2002). Episodic memory retrieval: an (event-related) functional neuroimaging perspective In: Parker A.E., et al. , editors. The Cognitive Neuroscience of Memory Encoding and Retrieval, 3–38, Hove: Psychology Press. [Google Scholar]
- Schacht A., Werheid K., Sommer W. (2008). The appraisal of facial beauty is rapid but not mandatory. Cognitive, Affective, & Behavioral Neuroscience, 8(2),132–42. [DOI] [PubMed] [Google Scholar]
- Schneider W., Eschman A., Zuccolotto A. (2002). E-Prime User’s Guide. Pittsburgh, PA: Psychology Software Tools, Inc. [Google Scholar]
- Schultz W. (2000). Multiple reward signals in the brain. Nature Reviews Neuroscience, 1(3),199–207. [DOI] [PubMed] [Google Scholar]
- Schupp H.T., Öhman A., Junghöfer M., Weike A.I., Stockburger J., Hamm A.O. (2004). The facilitated processing of threatening faces: an ERP analysis. Emotion, 4(2),189.. [DOI] [PubMed] [Google Scholar]
- Schupp H.T., Stockburger J., Codispoti M., Junghofer M., Weike A.I., Hamm A.O. (2007). Selective visual attention to emotion. Journal of Neuroscience, 27(5),1082–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinberger S.R., Huddy V., Burton A.M. (2004). N250r: a face-selective brain response to stimulus repetitions. Neuroreport, 15(9),1501–5. [DOI] [PubMed] [Google Scholar]
- Slater A., Bremner G., Johnson S.P., Sherwood P., Hayes R., Brown E. (2000). Newborn infants’ preference for attractive faces: the role of internal and external facial features. Infancy, 1(2),265–74. [DOI] [PubMed] [Google Scholar]
- Slater A., Von der Schulenburg C., Brown E., et al. (1998). Newborn infants prefer attractive faces. Infant Behavior and Development, 21(2),345–54. [Google Scholar]
- Symons D. (1979). The Evolution of Human Sexuality. New York: Oxford University Press. [Google Scholar]
- Tanaka J.W., Curran T., Porterfield A.L., Collins D.R. (2006). Activation of preexisting and acquired face representations: the N250 event-related potential as an index of face familiarity. Journal of Cognitive Neuroscience, 18(9),1488–97. [DOI] [PubMed] [Google Scholar]
- Thiruchselvam R., Blechert J., Sheppes G., Rydstrom A., Gross J.J. (2011). The temporal dynamics of emotion regulation: an EEG study of distraction and reappraisal. Biological Psychology, 87(1),84–92. [DOI] [PubMed] [Google Scholar]
- Thiruchselvam R., Hajcak G., Gross J.J. (2012). Looking inward shifting attention within working memory representations alters emotional responses. Psychological Science, 23(12),1461–6. [DOI] [PubMed] [Google Scholar]
- Thornhill R., Gangestad S.W. (1999). Facial attractiveness. Trends in Cognitive Sciences, 3(12),452–60. [DOI] [PubMed] [Google Scholar]
- Trujillo L.T., Jankowitsch J.M., Langlois J.H. (2014). Beauty is in the ease of the beholding: a neurophysiological test of the averageness theory of facial attractiveness. Cognitive, Affective, & Behavioral Neuroscience, 14(3),1061–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hooff J.C., Crawford H., van Vugt M. (2010). The wandering mind of men: ERP evidence for gender differences in attention bias towards attractive opposite sex faces. Social, Cognitive, and Affective Neuroscience, 6, 477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werheid K., Schacht A., Sommer W. (2007). Facial attractiveness modulates early and late event-related brain potentials. Biological Psychology, 76(1),100–8. [DOI] [PubMed] [Google Scholar]
- Wiese H., Altmann C.S., Schweinberger S.R. (2014). Effects of attractiveness on face memory separated from distinctiveness: evidence from event-related brain potentials. Neuropsychologia, 56, 26–36. [DOI] [PubMed] [Google Scholar]
- Wieser M.J., Gerdes A.B., Büngel I., Schwarz K.A., Mühlberger A., Pauli P. (2014). Not so harmless anymore: how context impacts the perception and electrocortical processing of neutral faces. NeuroImage, 92, 74–82. [DOI] [PubMed] [Google Scholar]
- Wilding E.L. (2000). In what way does the parietal ERP old/new effect index recollection? International Journal of Psychophysiology, 35(1),81–7. [DOI] [PubMed] [Google Scholar]
- Wilson T.D., Gilbert D.T. (2008). Explaining away: a model of affective adaptation. Perspectives on Psychological Science, 3(5),370–86. [DOI] [PubMed] [Google Scholar]
- Zajonc R.B. (1968). Attitudinal effects of mere exposure. Journal of Personality and Social Psychology, 9(2p2), 1.5667435 [Google Scholar]
- Zajonc R.B. (2001). Mere exposure: a gateway to the subliminal. Current Directions in Psychological Science, 10(6),224–8. [Google Scholar]