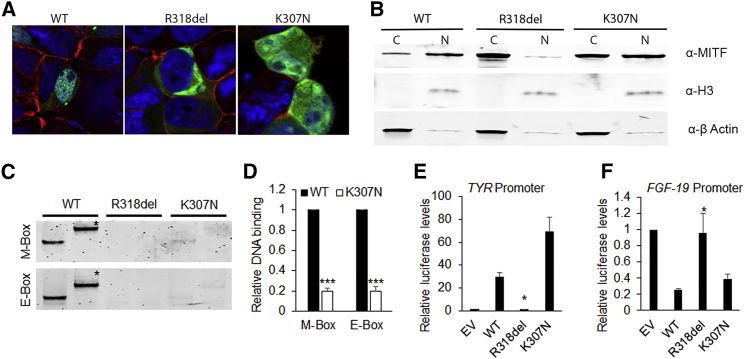
Figure 2.
Nuclear Migration, DNA Binding, and Promoter Regulatory Potential of WT and Mutant MITF-A Isoforms
HEK293 cells were cultured in DMEM with 10% FBS and 1% penicillin-streptomycin for 24 hr and then transiently transfected with fluorescent-protein-tagged wild-type (WT) or mutant MITF constructs with X-tremeGENE HP (Roche) according to the manufacturer’s instructions. Cells were fixed with 4% paraformaldehyde 24 hr after transfection, stained with Phalloidin (red) and DAPI, and photographed at 60× magnification with a confocal microscope.
(A) GFP-tagged WT MITF-A isoform was observed predominantly in the nucleus of cells. The p.Arg318del mutant was observed only in the cytoplasm, whereas p.Lys307Asn localized in both the cytoplasm and nucleus, suggesting some disruption of the nuclear migration ability (also see Movie S1).
(B) Subcellular localization of the mutants was further confirmed by western blot analysis of the cytoplasmic (C) and nuclear (N) fractions of cells transfected by WT and mutant MITF-A, in agreement with the fluorescence microscopy data. For protein blotting, cytoplasmic and nuclear fractions were separated with the Nuclear Extract Kit (Active Motif) according to the manufacturer’s instructions. 20 μg of protein (BCA Protein Assay Kit, Life Technologies) was separated by SDS-PAGE on polyacrylamide gels (Bio-Rad). Proteins were transferred onto polyvinylidene fluoride membranes (Bio-Rad) and probed with mouse monoclonal antibody to MITF (clone D5, Thermo Scientific). Rabbit polyclonal antibody to histone H3 and β-actin (Abcam) were used as loading controls. Membranes were then incubated with secondary antibodies (1:10,000; LI-COR) and then scanned with the Odyssey infrared scanner and analyzed with Image Studio Lite v.4.0 (LI-COR).
(C) Electrophoretic mobility shift assay (EMSA) was performed with DNA oligonucleotides harboring M-box and E-box consensus sequences (Table S2) labeled with IR700 dye (Integrated DNA Technologies) with the Odyssey Infrared EMSA Kit (LI-COR) as per the manufacturer’s instructions. WT and mutant MITF isoforms were in vitro translated with the SP6 TnT Quick Coupled Transcription/Translation System (Promega) as per the manufacturer’s instructions. 50 femtoM of duplex oligonucleotide was mixed with binding buffer (100 cmM Tris, 500 mM KCl, and 10 cmM DTT [pH 7.5]), poly(deoxyinosinic-deoxycytidylic), 1 M KCl, and the WT and/or mutant protein in vitro translation mix, incubated for 30 min at room temperature, and then separated on 5% TBE polyacrylamide gels (Bio-Rad) at 100V. The gels were scanned with an Odyssey scanner and analyzed with Image Studio Lite v.4.0 (LI-COR). M-box and E-box sequences showed strong DNA binding by WT MITF-A, no DNA binding by the p.Arg318del mutant, and significantly reduced binding by the p.Lys307Asn mutant. A supershift (∗), produced by MITF-specific antibody, confirmed the specificity of MITF and DNA binding (also see Figure S2).
(D) Quantification of the DNA binding by WT and p.Lys307Asn MITF-A, as calculated from three different trials.
(E and F) The Dual-Luciferase Reporter Assay System (Promega) was used to study the promoter regulatory potential of WT and mutant MITF-A isoforms. With X-tremeGENE HP (Roche), HEK239T cells (1 × 105 seeding density) were co-transfected 24 hr after seeding with 100 ng of MITF-A or -D isoforms, 200 ng of luciferase-promoter reporter construct, 1:1,000 Renilla luciferase vector (pRL Null Renilla), and empty vector to equalize the amount of transfected DNA. All transfections were performed in duplicate. Transfected cells were lysed 24 hr after transfection, and aliquots were used for determining firefly and Renilla luciferase activities in triplicate. Data were normalized to the activity of Renilla luciferase. All experiments were repeated three times. The WT and p.Lys307Asn mutant MITF-A activated the TYR promoter and repressed the FGF19 promoter. The p.Arg318del mutant showed no promoter regulatory potential. All comparisons are in reference to the WT MITF; error bars represent standard errors p values were calculated with the two-sided Student’s t test: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005.