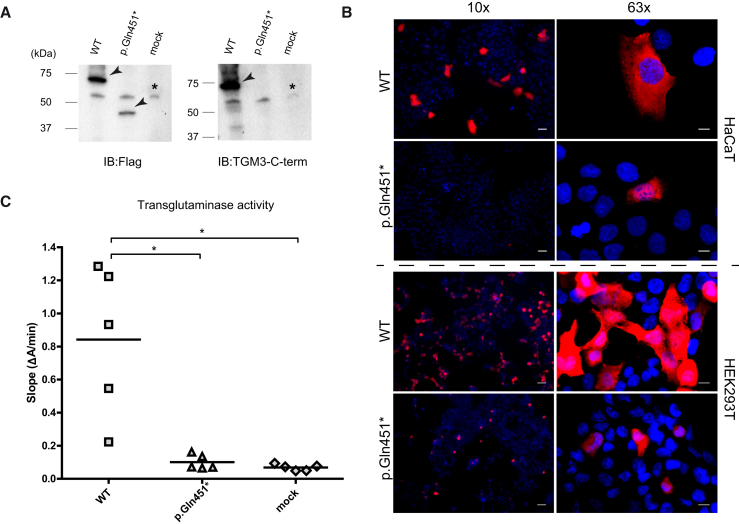
Figure 7.
WT and Mutant Forms of TGM3 Produced in HaCaT and HEK293T Cell Lines
(A) Immunoblotting analysis shows the translation of WT and truncated TGM3 in transiently transfected HaCaT cells, collected 48 hr post-transfection. Immunoblotting was performed with anti-Flag and anti-TGM3 antibodies. Antibody-specific bands showing the WT (∼70 kDa) and truncated TGM3 (∼40 kDa) are indicated with an arrow and a non-specific cross-reactive band around 55 kDa is indicated with an asterisk. The truncated TGM3 can be detected only with the antibody against the Flag epitope fused to the N terminus and not with the antibody against the C terminus of TGM3 as expected. Full-length blot images can be seen in Figure S9. Immunoblotting analysis with HEK293T cells is presented in Figure S7.
(B) Immunofluorescence analyses in HaCaT and HEK293T cells transiently transfected with WT and mutant TGM3 encoding constructs are presented by images captured with 10× and 63× objectives. The analyses show clear differences in the number of cells producing the WT and mutant TGM3 (10× images). Scale bars represent 50 μm and 10 μm for 10× and 63× objectives, respectively.
(C) Transglutaminase activity of WT and mutated TGM3 produced in HEK293T cells. Mock-transfected cell lysates were used as negative control. The transglutaminase activity, represented by the mean slope of 10 min long measurements from technical triplicates (given in Figure S8), is presented as a dot plot. The enzymatic activity assay was performed with samples emerging from five independent transfections. Horizontal lines represent the mean values (mean ± SD; WT, 0.84 ± 0.45; p.Gln451∗, 0.10 ± 0.04; mock, 0.07 ± 0.02). Results demonstrate that the mutated protein did not differ from the mock transfected negative control in terms of activity and WT TGM3 had a significantly higher activity in comparison to both (∗p < 0.05, Dunn’s test).