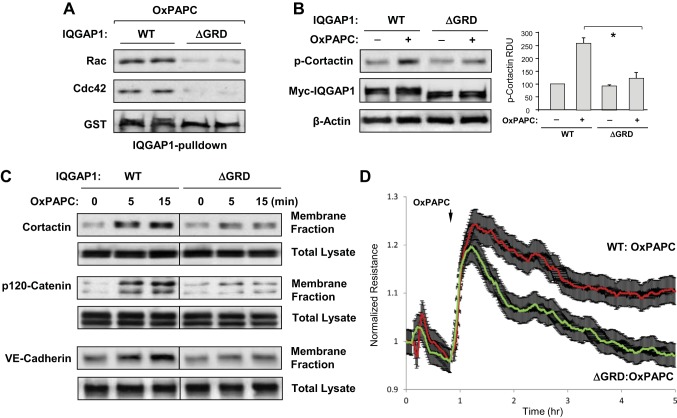
Fig. 4.
The GTPase regulatory domain (GRD) of IQGAP1 mediates OxPAPC-induced activation of Rac1/Cdc42 signaling and EC barrier enhancement. A: EC were stimulated with OxPAPC (10 μg/ml) for 5 min. EC lysates were incubated with agarose beads conjugated with IQGAP1-wild type (WT) or IQGAP1-ΔGRD. After being washed, immobilized Rac1 and Cdc42 were detected by immunoblotting. Probing the membranes with glutathione S-transferase (GST) antibody was used as a normalization control. Samples are shown in duplicate. B: OxPAPC-induced cortactin phosphorylation in EC expressing full-length IQGAP1 (IQGAP1-WT) or IQGAP1-ΔGRD was evaluated by Western blot with phospho-Y421-cortactin antibody. Expression of recombinant IQGAP1-WT and IQGAP1-ΔGRD was confirmed by probing the membrane with cMyc antibody. Equal protein loading was confirmed by probing for β-actin. Bar graphs depict quantitative analysis of Western blot data; n = 3; *P < 0.05 vs. IQGAP1-WT. C: HPAEC transfected with IQGAP1-WT or IQGAP1-ΔGRD were stimulated with OxPAPC, followed by fractionation assay. After isolation of cytosolic and membrane fractions, cortactin, p120-catenin, and VE-cadherin accumulation in the membrane fraction was monitored by Western blot. Protein content in corresponding total cell lysates was used as a normalization control. D: permeability measurements. EC transfected with IQGAP1-WT or IQGAP1-ΔGRD were stimulated with OxPAPC, and TER was monitored over time. Shown are normalized average TER values ± SE from 3 independent readings in 1 experiment; the data are representative of 4 independent experiments.