Fig. 3.
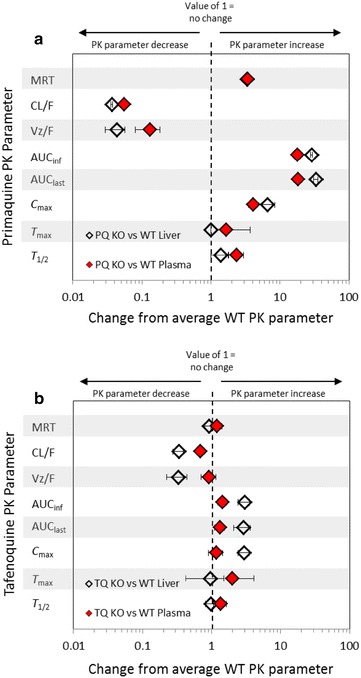
Relative fold changes of primaquine and tafenoquine CYP2D KO pharmacokinetic parameters from reference (WT parameters) in mouse plasma and liver. Indicated are the fold changes after the administration 20 mg/kg primaquine (PQ) and 20 mg/kg tafenoquine (TQ) in the CYP2D KO strain as compared to WT C57BL/6 mice. The fold change is indicated by the x-axis and the pharmacokinetic parameter on the y-axis. A value of one represents no relative change in the pharmacokinetic parameter between the KO and WT mice. The grey bars are provided for visual reference. The error shown is the standard deviation of relative fold changes for KO pharmacokinetic parameters as compared to WT means pharmacokinetic parameter values. PQ and TQ comparisons were conducted using experimental data generated at the WRAIR [26, 27]. WT C57BL/6 wild-type mice. CYP2D KO C57BL CYP2D6 knock-out mice. MRT mean resonance time. CL/F apparent total clearance of the drug from plasma after oral administration. Vz/F apparent volume of distribution during terminal phase after non-intravenous administration. AUCinf area under the plasma concentration-time curve from time zero to infinity. AUClast area under the plasma concentration-time curve from time zero to the time of last measurable concentration. Cmax: maximum (peak) plasma drug concentration. Tmax time to reachmaximum (peak) plasma concentration following drug administration. T1/2 elimination half-life
