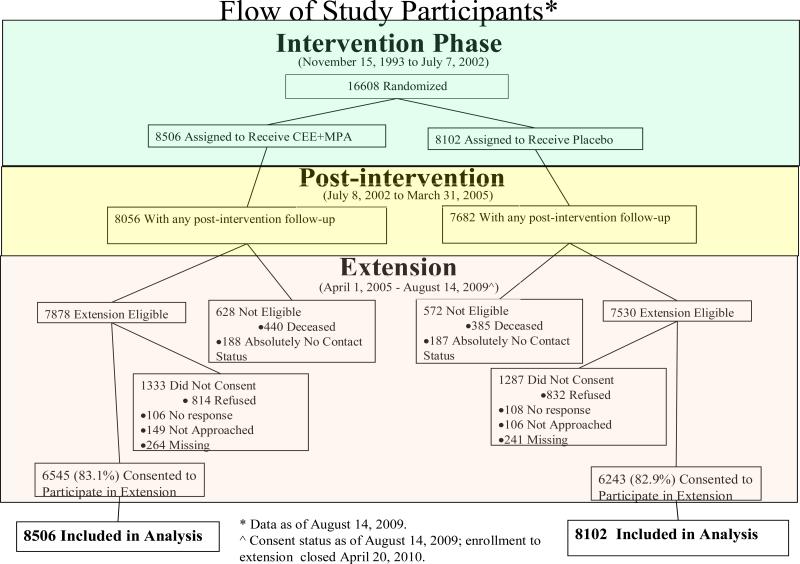
Figure 1.
Flow of study participants during the intervention, postintervention and extension phases. The post-intervention phase began on July 9, 2002 the day after participants were instructed to stop study medication use (conjugated equine estrogen plus medroxyprogesterone acetate or placebo) use. The post-intervention phase continues thru the original trial completion date (March 31, 2005). The extension phase began on April 1, 2005, and includes follow-up for participants who re-consented (83% of those eligible) thru August 14, 2009.