Fig. 7.
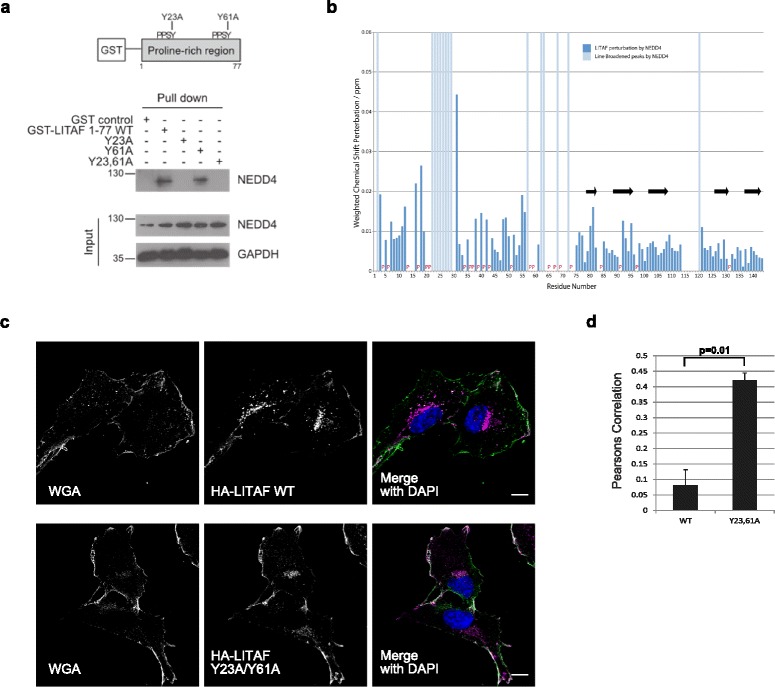
Recruitment of proteins to the proline-rich region determines the intracellular targeting of LITAF. a The first PPSY motif of LITAF (residues 20–23) interacts with the ubiquiting ligase, NEDD4. GST-LITAF 1–77 constructs (corresponding to the N-terminal proline-rich region), were recombinantly expressed and purified from E. coli, before incubation with cellular lysates prepared from retinal pigment epithelial (RPE) cells. A pull-down experiment was performed and the interacting proteins were separated by SDS-PAGE followed by western blotting. b The chemical shift perturbations seen in the LITAF Δ114–139 construct following incubation with recombinantly expressed NEDD4 596–944 (containing the 4 WW domains). A number of peaks are line-broadened indicating the interaction of the NEDD4 WW domains with these discrete regions of the LITAF proline-rich N-terminus. The interaction of NEDD4 with the proline-rich region had no effect on the C-terminal LITAF domain. c Representative images of confocal immunofluorescence microscopy of RPE cells stably expressing HA-LITAF wild type (WT) and HA-LITAF Y23A/Y61A. While HA-LITAF WT predominantly targets to endocytic vesicles, HA-LITAF Y23A/Y61A is mislocalised to the plasma membrane, labelled with wheat germ agglutinin (WGA). The merged images contain WGA coloured green and HA-LITAF coloured purple with areas of colocalisation appearing white. Scale bars denote 10 μm. d The colocalisation of HA-LITAF constructs with WGA was quantified using Volocity (Perkin Elmer) and a Pearson’s Correlation calculated; 10–13 images (each containing between four and five cells per field of vision) were taken from three biological replicates per cell line stably expressing the LITAF constructs. The Pearson’s correlation was calculated by the Volocity software with the threshold applied as previously described [87] (see Additional file 14 for individual data values). The graph shows the mean values with error bars denoting SEM. A P value of 0.01 was calculated using an unpaired two-tailed t-test. Significantly greater amounts of the HA-LITAF Y23,61A protein colocalised with WGA compared to wild type, confirming mislocalisation of the mutated construct towards the plasma membrane
