Abstract
Cystic fibrosis (CF) is a life-shortening disease caused by the mutations that generate nonfunctional CF transmembrane conductance regulator (CFTR) protein. A rare serine-to-tyrosine (S1045Y) CFTR mutation was earlier reported to result in CF-associated fatality. We identified an African-American patient with the S1045Y mutation in CFTR, as well as a stop-codon mutation, who has a mild CF phenotype. The underlying mechanism of CF caused by S1045Y-CFTR has not been elucidated. In this study, we determined that S1045Y-CFTR exhibits twofold attenuated function compared with wild-type (WT)-CFTR. We report that serine-to-tyrosine mutation leads to increased tyrosine phosphorylation of S1045Y-CFTR, followed by recruitment and binding of E3-ubiquitin ligase c-cbl, resulting in enhanced ubiquitination and passage of S1045Y-CFTR in the endosome/lysosome degradative compartments. We demonstrate that inhibition of tyrosine phosphorylation partially rescues S1045Y-CFTR surface expression and function. Based on our findings, it could be suggested that consuming genistein (a tyrosine phosphorylation inhibitor) would likely ameliorate CF symptoms in individuals with S1045Y-CFTR, providing a unique personalized therapy for this rare CF mutation.
Keywords: cystic fibrosis transmembrane conductance regulator, rare cystic fibrosis mutations
approximately one in every 3,500 children born in the United States inherits cystic fibrosis (CF) (7). Although CF affects multiple organs, most debilitating symptoms occur in the lung with recurrent episodes of bacterial infections and perpetual decline in lung function (6, 21, 26). CF develops due to biallelic inheritance of loss-of-function mutations in the gene encoding for the CF transmembrane conductance regulator (CFTR) membrane protein. To date, ∼2,000 mutations have been identified in the cftr gene (http://www.genet.sickkids.on.ca/cftr). The CFTR protein is a member of the ATP-binding cassette transporter family that actively transports chloride ions (Cl−) in the lumen when stimulated by cyclic nucleotides (cAMP or cGMP) and this, together with sodium ions, drives the osmotic flow of water (2, 9, 12, 13). Defects in CFTR synthesis or function perturb the water-driving force, and the epithelial surfaces lining the lung, gastrointestinal tract, and pancreas become dehydrated, resulting in the mucus secretions becoming sticky and building up on these epithelial surfaces. This defect manifests as defective mucociliary clearance in the lungs with 100% penetrance in CF, meconium ileus (obstructed gut) in the gut in 10% of CF infants, and pancreatic insufficiency in 80–90% of the CF patient population (25). CF-causing CFTR mutations are broadly categorized into five classes depending on the spectra of causes that affect synthesis and/or function (28). Class I CFTR mutations (e.g., G542X) lead to severed protein synthesis due to the presence of a premature stop codon, class II mutations (e.g., the most common CF-causing mutation, ΔF508) have folding or maturation defects, both of which can result in premature CFTR degradation, class III mutations (e.g., G551D) cause defective gating and inability to pump Cl− in response to cAMP, class IV mutations (e.g., R117H) affect chloride conductance due to defects in the channel pore, and class V mutations (e.g., 3849+10KbC>T) change code for splice mutants, leading to an overall reduction in normal CFTR synthesis (22). On the basis of molecular defects, mutations in classes I-III would cause more severe phenotypic consequences (22, 28). Conversely, class IV and V mutants may have residual CFTR function, thus leading to a milder CF phenotype. Additional defect (class VI) in CFTR function is related to poor retention of the protein at the plasma membrane connected to multiple factors, such as for rescued F508del-CFTR mutant emanating from thermodynamic and kinetic destabilization of NBD1 with the mutation, misfolding-driven internalization, and poor abilities to engage in protein-protein interactions (4, 10, 19, 24).
Surprisingly, CFTR trafficked normally to the plasma membrane can become rapidly endocytosed (5–10%/min) (24). A tyrosine-based (YXXϕ) internalization motif 1424YDSI in CFTR is conserved across species and mediates CFTR endocytosis by adaptor protein complex-2 clathrin-mediated mechanisms (1, 18). Recent findings imply that ubiquitin can play a major role in regulating the fate of membrane proteins (17, 24). Membrane surface receptors such as epidermal growth factor receptor (EGFR) may be decorated with poly- or monoubiquitin, with the former driving to proteasomal degradation and the latter directing it to lysosomal degradation (8, 11, 23). E3 ubiquitin ligase casitas B-lineage lymphoma proto-oncogene (c-cbl) is an important player in tagging receptor tyrosine kinases with ubiquitin, including activated/phosphorylated EGFR, facilitating its recruitment into lysosomes (23). The association of c-cbl with EGFR and subsequent ubiquitination likely occurs at the plasma membrane before internalization, but it seems to function as a sorting signal for an endocytic fate postinternalization, and c-cbl remains associated with EGFR throughout the endocytic pathway until the final fate is decided (11). c-cbl harbors a tyrosine kinase-binding domain and binds EGFR in its activated state (pY1045 EGFR). It has been suggested that c-cbl binds wild-type (WT)-CFTR, labeling it with ubiquitin in endosomes and channeling it to lysosomes for degradation (27). By reducing c-cbl expression, an increase in CFTR-mediated Cl− currents was observed (27). The same group demonstrated that c-cbl can affect ΔF508 CFTR surface expression (5). The consensus binding site for c-cbl is (D/E)XpYXXXφ (where φ is a hydrophobic amino acid; usually proline).
We identified an African-American patient with mild CF phenotype carrying two CFTR mutations: c.2885C>A (p.Ser926X) and c.3134C>A (p.Ser1045Tyr). To determine the molecular mechanism rendering S1045Y CFTR mutation as pro-CF, we studied the flanking sequence surrounding the tyrosine mutation and determined that the serine (Ser)-to-tyrosine (Tyr) alteration at position 1045 changes the context of the sequence into a putative c-cbl-binding site. We hypothesized that the mutant protein might therefore acquire the fate of activated EGFR upon tyrosine phosphorylation and becomes a c-cbl target, leading to ubiquitination and a major proportion of the tyrosine-phosphorylated CFTR ultimately residing in endosomes/lysosomes.
MATERIALS AND METHODS
Patient Studies
Patient studies were approved under Institutional Review Board no. 13-02779-XM.
Generation of the S1045Y CFTR Mutant
Ser to Tyr mutation was generated using the QuikChange II Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA) to convert cytosine-3134 to adenine-3134 (c.3134C>A) and using FLAG-WT-CFTR cDNA as a template. Stable cells expressing FLAG-WT and FLAG-S1045Y-CFTR were generated by transducing HEK 293 and CFBEo− cells with the lentiviral particles carrying WT-CFTR and S1045Y-CFTR, with subsequent selection of positive clones on 10 μg/ml puromycin for 1 wk and maintenance on 2 μg/ml puromycin.
Reagents
Tyrosine kinase inhibitors ruxolitinib, lapatinib, and imatinib were purchased from Selleck Chem (Houston, TX). Cycloheximide (CHX) and genistein were obtained from Sigma-Aldrich (St. Louis, MO). Forskolin was purchased from Tocris Bioscience (Bio-Techne, Minneapolis, MN).
Immunoblotting
HEK 293 parental cells (no CFTR), HEK 293-FLAG-WT-CFTR cells, and HEK 293-FLAG-S1045Y-CFTR cells were maintained in 3 × 100 mm dishes for each at 37°C until 100% confluent. To induce a tyrosine phosphorylation cascade, cells were subjected to 10 μM H2O2 and 20 μM sodium orthovanadate for 60 min at 37°C. After incubation, cells were lysed in 3 ml RIPA lysis buffer [50 mM Tris·HCl, 150 mM NaCl, 1% Nonidet P-40 (NP-40), 0.5% sodium deoxylcholate, 0.1% SDS, and 1 mM EDTA + protease inhibitor cocktail (1 μM aprotonin, 1 μM leupeptin, 1 μM pepstatin, 1 mM phenylmethylsulfonyl fluoride)]/dish. Subsequently, 50 μl FLAG-beads suspension (Sigma-Aldrich) were added and mixed overnight at 4°C. Beads were washed three times, and samples were eluted with glycine buffer containing 0.2% Triton X-100 (pH 2.2), followed by immediate neutralization with 1.5 M Tris-Cl (pH 8.8). Eluates were separated on 4–15% gels and probed with rabbit polyclonal c-cbl antibody (Cell Signaling Technology, Danvers, MA) prepared in 5% blotting-grade milk in TBS-Tween.
To check for ubiquitination parental, FLAG-WT, FLAG-S1045Y-CFTR-expressing HEK 293 cells were grown in 60-mm dishes to 100% confluence and lysed in PBS 0.2% Triton X-100 buffer containing protease inhibitors. Proteins were immunoprecipitated with 20 μl FLAG beads mixed overnight at 4°C and eluted in 50 μl glycine buffer as mentioned before. The proteins were probed with rabbit Ub antibody (Cell Signaling Technology) diluted in 5% BSA TBS-Tween. To detect CFTR protein in Western blots, mouse monoclonal antibody M3A7 (Thermo Scientific, Waltham, MA) prepared in 5% blotting-grade milk in TBS-Tween was used. To detect interaction with Janus kinase 1 (JAK1), similarly prepared cells were probed with rabbit polyclonal JAK1 (Cell Signaling Technology) prepared in 5% blotting-grade milk in TBS-Tween.
To check for tyrosine-phosphorylated proteins, HEK 293 cells grown to confluence in 60-mm dishes were treated with 100 μM H2O2 and 2 mM sodium orthovanadate for 15 min at 37°C and lysed and immunopreciptated with FLAG beads. CFTR purified on the FLAG beads was glycine eluted and probed with pY horseradish peroxidase (HRP) antibody (Thermo Scientific) prepared in 5% BSA TBS-Tween.
Immunofluorescence
HEK 293 cells expressing WT- or S1045Y-CFTR protein grown to 50% confluence were washed with PBS two times and fixed in 4% paraformaldehyde for 15 min, followed by a permeabilization step using PBS 0.3% Triton X-100 for 15 min. Cells were then blocked in 4% BSA PBS-Tween for 2 h at room temperature (RT) and incubated with rabbit R3194 CFTR antibody (1:100) overnight at 4°C. After incubation, cells were washed three times with PBS 0.05% Tween (5 min each wash). Alexa-fluor 488 rabbit secondary antibody (1:500) was added to the cells for 1 h, and cells were washed three times with PBS 0.05% Tween (5 min each wash). In the final step, cells were mounted in Vectashield fluorescence mounting media containing DAPI. Images were captured using an Olympus FV1200 confocal microscope.
CFTR Endocytosis Assay
HEK 293 cells expressing WT- or S1045Y-CFTR protein were grown in 60-mm dishes to confluence. Cells were washed two times with prewarmed PBS, pH 7.4, containing Ca2+ and Mg2+ and subsequently washed one time with ice-cold PBS, pH 8.0, without Ca2+ and Mg2+. Washed cells were incubated with 1 mg/ml cell-impermeant EZ-Link-Sulfo-NHS-S-S-Biotin (Thermo Scientific) with a cleavable disulfide bond prepared in PBS, pH 8.0, to label the surface proteins, including CFTR, on ice to prevent any endocytosis at this stage. A set of dishes was maintained on ice, therefore corresponding to no endocytosis, to determine the total amount of labeled surface CFTR and normalize the endocytosed protein. Separately, cells labeled with biotin kept on ice were switched to 37°C to trigger endocytosis and followed for 5 min. Later, these cells were switched back to 4°C, and biotin was reductively cleaved from the remaining surface proteins by incubating the cells two times for 15 min with stripping buffer, pH 8.6 [100 mM 2-mercaptoethanesulfonic acid (MESNA), 50 mM Tris, 100 mM NaCl, 1 mM EDTA, and 0.2% BSA], with no shaking. Cells were subsequently rinsed one time with PBS 1% BSA and incubated on ice for 15 min. Next, cells were lysed in NP-40 lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 0.1% NP-40). After protein concentrations were measured and the protein amounts for each sample were normalized, 30 μl streptavidin-conjugated resin were added to each sample and allowed to mix overnight at 4°C. After incubation, samples were washed three times with the NP-40 lysis buffer and eluted in 5× sample buffer. Samples were run on 8% polyacrylamide gels and immunoblotted with mouse monoclonal M3A7 CFTR antibody. Levels of CFTR protein were calculated as mean gray value using ImageJ.
Biotinylation of Surface CFTR
HEK 293 cells expressing WT- or S1045Y-CFTR protein were grown in 60-mm dishes. To monitor the effect of tyrosine phosphorylation on CFTR endocytosis, cells were subjected to 10 μM H2O2 and 20 μM sodium orthovanadate for 15 or 60 min at 37°C with no treatment controls in parallel. After incubation, cells were put on ice, washed two times with ice-cold PBS, pH 7.4, containing Ca2+ and Mg2+, and washed one time with ice-cold PBS, pH 8.0, without Ca2+ and Mg2+. Cells were then incubated with 1 mg/ml membrane-impermeant EZ-Link Sulfo-NHS-LC-Biotin (Thermo Scientific) prepared in PBS, pH 8.0, to label the surface proteins, including CFTR, on ice to prevent endocytosis. Cells are subsequently rinsed one time with PBS 1% BSA and incubated on ice for 15 min. Next, cells were lysed in NP-40 lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, and 0.1% NP-40). After protein concentrations were measured and the protein amounts for each sample were normalized, 30 μl streptavidin agarose were added to each sample and allowed to mix overnight at 4°C. Samples were then washed three times with the NP-40 lysis buffer and eluted in 5× sample buffer. Samples were run on 8% polyacrylamide gels and immunoblotted with mouse monoclonal M3A7 CFTR antibody. Levels of CFTR protein were calculated as mean gray value using ImageJ.
Iodide Efflux Assay
HEK 293 cells stably expressing WT-CFTR and S1045Y-CFTR were grown on poly-l-lysine-coated 60-mm dishes to 100% confluence. Briefly, cells were loaded for 60 min at RT with loading buffer [136 mM NaI prepared in the buffer with the following composition (in mM): 137 NaCl, 4.5 KH2PO4, 1 CaCl2, 1 MgCl2, 10 glucose, and 5 HEPES, pH 7.2]. Extracellular NaI was washed away thoroughly (5–7 times) with efflux buffer (136 mM NaNO3 prepared in the buffer as mentioned before replacing 136 mM NaI in the loading buffer) and then cells were equilibrated for 1 min each and aliquots were collected for four times to establish a stable baseline in the efflux buffer alone. After basal equilibrium with the nitrate buffer was established, cells were treated with CFTR agonists (10 μM forskolin and 100 μM IBMX) prepared in the nitrate buffer, and aliquots were collected for six times at 1-min intervals. The iodide concentration of each aliquot collected was determined using an iodide-sensitive electrode (Thermo Scientific) and converted to iodide content (nmol/min) as described previously (16).
Short-Circuit Current Measurements
Polarized CFBEo− monolayers were grown on Costar Transwell permeable supports (Cambridge, MA; filter diameter 6.5 mm) until the monolayers reached both confluency and transepithelial resistance >800 Ω. The Transwells were subsequently mounted in an Ussing chamber maintained at 37°C. Short-circuit current (Isc) was measured as described previously (9). Epithelial cells were bathed in Ringer solution (in mM; basolateral side: 140 NaCl, 5 KCl, 0.36 K2HPO4, 0.44 KH2PO4, 1.3 CaCl2, 0.5 MgCl2, 4.2 NaHCO3, 10 HEPES, and 10 glucose, pH 7.2, [Cl−] = 149) and low-Cl− Ringer solution (in mM; apical side: 133.3 sodium gluconate, 2.5 NaCl, 0.36 K2HPO4, 0.44 KH2PO4, 5.7 CaCl2, 0.5 MgCl2, 4.2 NaHCO3, 10 HEPES, and 10 mannitol, pH 7.2, [Cl−] = 14.8) and saturated with 95% O2 and 5% CO2. A 2-mV pulse was applied every 1 min throughout the experiment to check for the integrity of the epithelia. After stabilization of basal Isc, cells were treated with forskolin on the apical side.
To test the effect of tyrosine kinase inhibitors on S1045Y-CFTR function, S1045Y-CFTR-expressing CFBEo− cells were preincubated with 10 µM ruxolitinib, 50 μM genistein, and 0.5 μM lapatinib for 15 min before addition of CFTR agonists. At the end of every Isc measurement, CFTR inhibitor (CFTRinh-172; 20–50 μM) was added to the apical side at the end of the experiment to verify that the Isc were CFTR dependent.
Surface-Labeling Assay
HEK 293 cells expressing FLAG-WT-CFTR, S1045Y-CFTR were grown in poly-l-lysine-coated 12-well plates until 100% confluent. In an initial step, cells were incubated with 100 μg/ml CHX for 3 h at 37°C to eliminate any contribution from newly synthesized protein. Afterward, tyrosine kinase inhibitors were added, and cells were incubated for 60 min at 37°C. Cells were then washed, fixed with 3.7% formaldehyde for 10 min, blocked with 1% BSA for 90 min, and treated with α-FLAG HRP (0.2 μg/ml) for 90 min. The HRP substrate 1-Step Ultra TMB (Pierce) was then added to the dishes and observed for color intensity. The reaction was stopped by adding an equal amount of 2 N H2SO4, and absorbance was read at 450 nm.
Statistical Analysis
Results are presented as means ± SE for the indicated number of experiments. Statistical analysis was performed using Student's t-test. A value of P < 0.05 was considered statistically significant.
RESULTS
Homozygous State of S1045Y CFTR Can Cause CF
It is important for this study that we establish the clinical basis of investigating S1045Y-CFTR mutation. We identified an individual who is a carrier of S1045Y, with the mutation in the second allele causing a synthesis block in CFTR, and who suffers from mild CF. Individuals who carry ΔF508-CFTR with one normal copy of CFTR are CF asymptomatic, and there needs to be two defective copies of that mutant CFTR protein to inherit CF. This supports that the S1045Y mutation in CFTR can potentially lead to certain CFTR-associated defects. A S1045Y mutation in CFTR on both alleles was previously reported in a Galician female who had confirmed CF (20). Therefore, S1045Y-CFTR could cause severe CF in its homozygous state. We present here a previously undocumented brief clinical summary of a S1045Y-CFTR carrier with mild CF.
Case summary.
The patient is an African-American full-term male born in 2012 in Mississippi, to a 20-yr-old mother, weighing 7 lb 7 oz. The State of Mississippi newborn screen was positive for elevated immunotrypsinogen (IRT; 244.5 ng/ml) with no identified CFTR mutations. Repeat screen 1 mo later showed persistent elevation of IRT (117.5 ng/ml). Therefore, sweat chloride testing was performed. Initial sweat test revealed intermediate values (right arm 43 mmol/l, left arm 52 mmol/l), and repeat testing showed mildly positive values (right arm 61 mmol/l, left arm 51 mmol/l). There was no family history of CF or other genetic or lung diseases. Although the patient did not have any respiratory symptoms or malabsorptive symptoms, he was then referred to the University of Tennessee Cystic Fibrosis Care Center for further evaluation (Table 1).
Table 1.
Clinical summary of patient carrying one S1045Y CFTR mutation
| Paramaters | Patient's Profile |
|---|---|
| Pancreatic insufficiency at birth | Present |
| Sweat chloride | Intermediate (right arm 43 mmol/l, left arm 52 mmol/l) |
| Weight gain | 3rd-10th percentile for weight, with an average weight gain of 5 g/day |
| Pulmonary symptoms | Continuous rhinorrhea and worsening cough at night at 9 mo of age |
| Respiratory flora | Klebsiella pneumoniae, Enterobacter cloacae, and Chryseobacterium meningosepticum |
| CFTR mutation | p.Ser926X and p.Ser1045Tyr Mother is a p.Ser1045Tyr carrier |
CFTR, cystic fibrosis transmembrane conductance regulator.
At initial evaluation, CF genotyping was obtained (Ambry Genetics), and results revealed two CFTR mutations: c.2885C>A (p.Ser926X) and c.3134C>A (p.Ser1045Tyr). Mother's genotype was also obtained and showed she carried only c.3134C>A (p.Ser1045Tyr), confirming the two rare mutations were in trans position. The patient was initially placed on pancreatic enzymes; however, stool elastase results (>200 ng/ml) later revealed pancreatic sufficiency, and these were discontinued. Despite normal pancreatic function at the age of 7 mo, the patient began showing signs of poor weight gain that continued until ∼9 mo of age. He was found to be in the 3rd-10th percentile for weight, with an average weight gain of 5 g/day. A repeat stool elastase performed at 9 mo of age confirmed pancreatic sufficiency (elastase >500 ng/ml). Nutrition services provided education and resources to aid the mother in an appropriate feeding regimen with a high-protein, high-calorie diet that enhanced his weight gain to the 50th percentile successfully.
At 9 mo of age, he began showing signs of respiratory illness. During a routine maintenance visit, the mother noted that his pulmonary symptoms had worsened to include continuous rhinorrhea and worsening cough at night. She denied any associated fever or wheezing. Chest radiograph showed peribronchial thickening, and, because of the persistent respiratory symptoms, he was admitted for intravenous antibiotics. Respiratory (throat) culture revealed Klebsiella pneumoniae, Enterobacter cloacae, and Chryseobacterium meningosepticum. He was initially started on intravenous tobramycin and piperacillin-tazobactam, which was later switched to oral trimethoprim-sulfamethoxazole to complete a full 14-day course. Since that time, he has only required one additional course of oral antibiotics for congestion and cough.
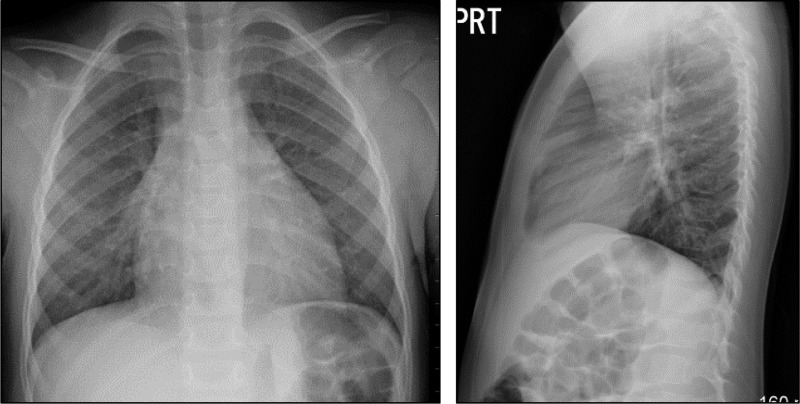
The patient has continued follow-up in the CF clinic regularly and continued to show good weight gain. His only medication consists of a daily multivitamin, and no respiratory treatments have been initiated with excellent weight gain off of pancreatic enzyme replacement. Subsequent respiratory cultures have shown methicillin-sensitive Staphylococcus aureus (MSSA) and, most recently, only normal flora. The chest X-ray of the patient at age 3 yr was read as showing minimal to no bronchial thickening (Fig. 1).
Fig. 1.
Posterior-anterior (PA) (left) and lateral (right) chest radiograph of an African-American cystic fibrosis (CF) patient carrying c.2885C>A (p.Ser926X, a stop codon mutation) and c.3134C>A (p.Ser1045Tyr) mutations. Chest radiograph shows minimal to no bronchial thickening.
Previous studies estimate that correction of the CF phenotype requires restoration of mutant CFTR function to 25–35% of the WT-CFTR activity (1, 15). Since this patient had a stop codon mutation, which encodes a truncated CFTR protein, and the S1045Y mutation with a CF phenotype, and due to a prior study reporting that an individual homozygous for S1045Y had severe CF disease (20), we conducted biochemical studies to unveil the molecular characteristics of S1045Y-CFTR.
S1045Y-CFTR Exhibits a 50% Reduction in Channel Function Compared With WT-CFTR
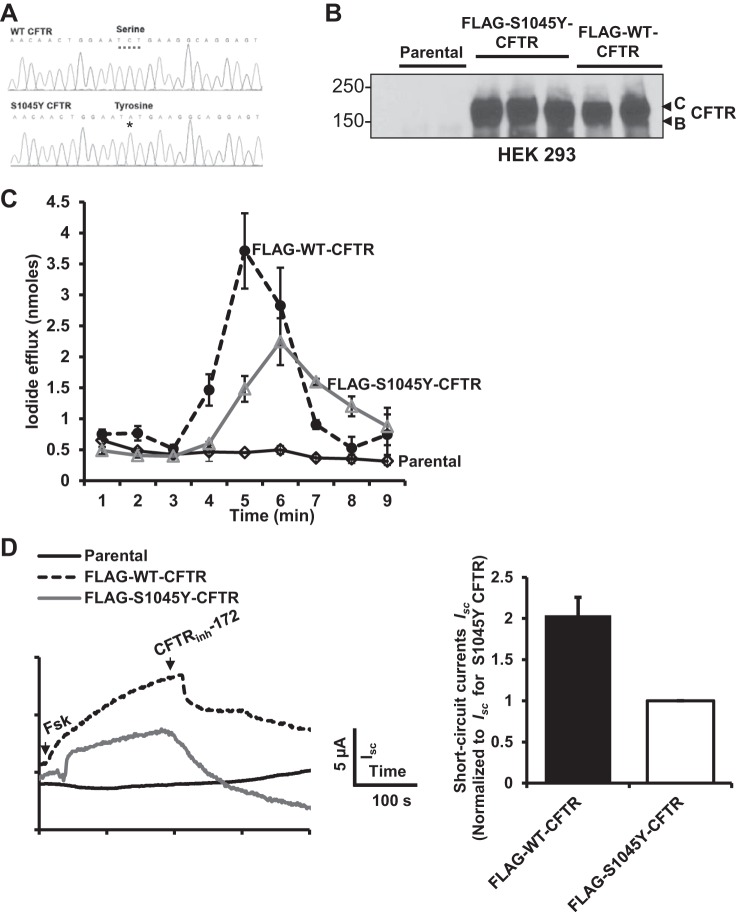
To study the molecular profile of S1045Y-CFTR, we used site-directed mutagenesis to generate a Ser-to-Tyr mutation at position 1045 in CFTR protein using WT-CFTR as a template (Fig. 2A). Stable HEK 293 cell lines carrying FLAG-WT-CFTR or FLAG-S1045Y-CFTR were generated and tested for CFTR protein expression and channel function. S1045Y-CFTR protein was expressed similarly as WT-CFTR, with normal band C and B configurations, suggesting that S1045Y-CFTR traffics properly to the plasma membrane (Fig. 2B). Functional analysis of CFTR revealed a 50% reduction of function with the S1045Y mutation compared with WT-CFTR using an iodide efflux assay (Fig. 2C). We also generated CFBEo− cells carrying the S1045Y mutation (Fig. 2D) and monitored CFTR channel function in polarized cells mounted in an Ussing chamber, which is a more physiologically relevant system to study CFTR function. We observed a 50% reduction in the function of S1045Y-CFTR compared with WT-CFTR.
Fig. 2.
S1045Y-cystic fibrosis transmembrane conductance regulator (CFTR) exhibits reduced function compared with wild-type (WT)-CFTR. A: DNA chromatogram to depict generation of Ser-to-Tyr mutation by conversion of cytosine to adenine nucleotide at position 3134. B: Western blot showing that S1045Y-CFTR has similar band C and B configurations as those of WT-CFTR. C: iodide efflux measurements in HEK 293 cells showing that S1045Y-CFTR exhibits ∼50% channel activity of normal CFTR. D: left, representative CFTR-mediated short-circuit currents (Isc) in CFBEo− cells. Right, data quantification from experiments as shown on left. Data were normalized to S1045Y-CFTR-mediated Isc from n = 3 independent experiments.
S1045Y-CFTR Exhibits Accelerated Internalization Compared With WT-CFTR
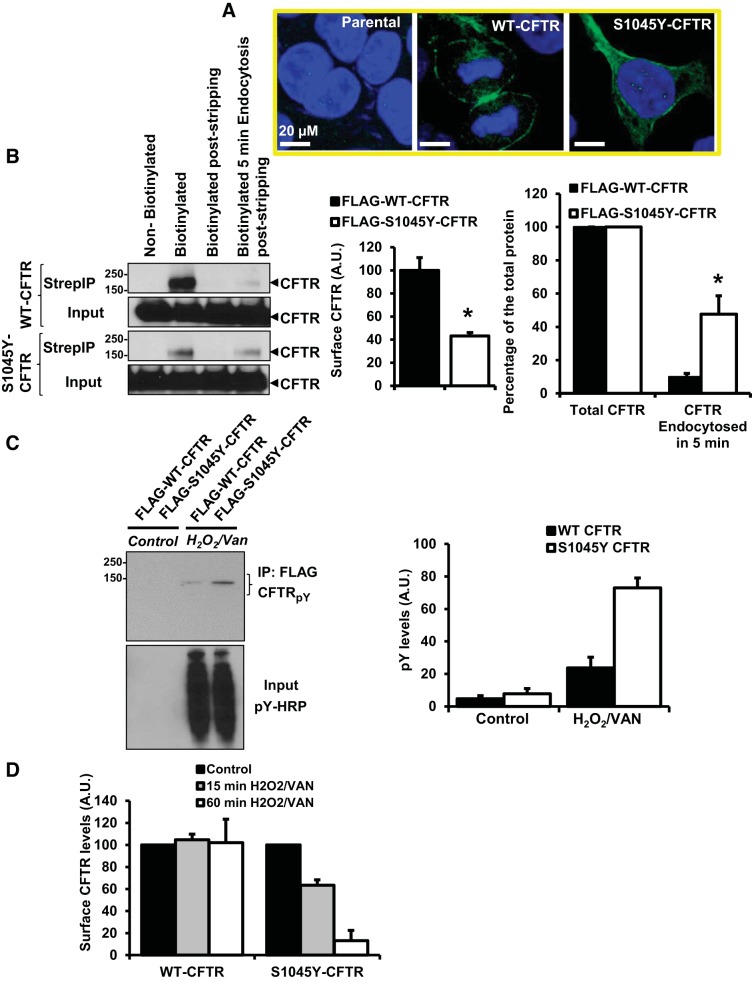
Because of the introduction of a Tyr residue in the S1045Y mutation and the role of Tyr-based endocytic motifs, we were interested in determining whether this mutation would alter the rate of internalization of the mutant protein. Using CFTR-specific immunostaining, we observed a significant intracellular presence of S1045Y-CFTR compared with WT-CFTR (Fig. 3A). This provided a first indication of S0145Y-CFTR being unable to remain at the cell surface as well as WT-CFTR. To further validate this observation, we used a cleavable cell-impermeant EZ-Link-Sulfo-NHS-S-S-Biotin to label surface proteins, including CFTR, and follow their endocytic fate. In this assay, cells labeled and maintained on ice are at a state of no endocytosis, which would determine the total amount of labeled surface CFTR for normalizing the endocytosed protein. Separately, cells labeled with this biotin on ice are switched to 37°C to trigger endocytosis and followed for 5 min. Cells are then switched back to 4°C, and biotin is reductively cleaved from the remaining surface proteins. We observed that the surface levels of S1045Y-CFTR are twofold lower compared with WT-CFTR, and ∼47% of S1045Y-CFTR was internalized in 5 min compared with ∼10% for WT-CFTR (Fig. 3B). Thus, the Tyr mutation at 1045 predisposes CFTR to rapid endocytosis.
Fig. 3.
S1045Y-CFTR exhibits accelerated internalization compared with WT-CFTR. A: confocal images depicting CFTR immunostaining (green) in HEK 293 parental cells and HEK 293 cells expressing WT-CFTR or S1045Y-CFTR. Nuclei were stained with DAPI (blue). B: left, Western blot of surface biotinylation assay to monitor WT- and S1045Y-CFTR protein under conditions of no endocytosis and no surface protein stripping (surface CFTR), no endocytosis with surface protein stripping, and endocytosis for 5 min and surface protein stripping. A no-biotinylation control was included in the experiment. Bar graphs show the quantitation of the surface biotinylation experiment for total surface CFTR (left) and endocytosed fraction of CFTR in 5 min normalized to total surface CFTR protein (right). *P < 0.05 for n = 4 independent experiments determined using Student's t-test. AU, arbitrary units; Strep, streptavidin. C: Western blot showing tyrosine phosphorylation of CFTR protein immunopreciptated from HEK 293 cells using FLAG-resin that could be clearly visualized using H2O2/vanadate (VAN) challenge. Tyrosine phosphorylation was detected using pY-horseradish peroxidase (HRP) antibody. Input refers to total cell lysate demonstrating induction of tyrosine phosphorylation following treatment with H2O2/vanadate. Bar graph on the right shows quantitation of the pY-CFTR ± H2O2/vanadate from n = 3 independent experiments. D: quantitation of WT- and S1045Y-CFTR surface protein levels following surface biotinylation assay with no endocytosis or membrane permeablization ± H2O2/vanadate for 15 and 60 min; n = 3–4 independent experiments. S1045Y-CFTR surface levels rapidly decline upon induction of tyrosine phosphorylation.
After we determined that S1045Y-CFTR is synthesized normally, traffics properly to the membrane, but becomes rapidly endocytosed, we further explored the molecular changes that would trigger this process. We found that the introduction of Tyr turns the sequence context into a putative binding site of the E3 ubiquitin ligase c-cbl (KQLEpYEGRSP) in its phosphorylated state. We therefore hypothesized that a phosphorylation event could render S1045Y-CFTR endocytic, and it may not be typical of the COOH-terminal Tyr motif-based endocytosis of CFTR. We used H2O2 (10 μM)/vanadate (0.02–1 mM) to accumulate Tyr phosphorylation in HEK 293 cells overexpressing FLAG-tagged WT-CFTR and S1045Y-CFTR. To test whether the Tyr mutation becomes phosphorylated in S1045Y-CFTR, we immunoprecipitated FLAG-tagged WT- and S1045Y-CFTR proteins from cells with or without H2O2/vanadate treatment using FLAG-antibody-conjugated resin. The immunoprecipitated proteins (CFTR enriched) were probed with HRP-conjugated phosphotyrosine antibody. We observed that S1045Y-CFTR exhibited three- to fourfold enhanced tyrosine phosphorylation corresponding to 1045Y compared with WT-CFTR with H2O2/vanadate challenge (Fig. 3C). To determine whether enhanced tyrosine phosphorylation of S1045Y-CFTR predisposes the protein to a rapid endocytic process, we used a surface biotinylation assay. HEK 293 cells overexpressing FLAG-tagged WT-CFTR or S1045Y-CFTR were subjected to 15- and 60-min treatments with H2O2/vanadate at 37°C and subsequently labeled with cell-impermeable biotinylating reagent NHS-LC-Biotin. We observed that a 15-min Tyr phosphorylation trigger reduced S1045Y-CFTR surface levels by 40%, and only 10% of S1045Y-CFTR remained at the surface after 60 min of the treatment. The surface levels of WT-CFTR remained intact under these treatment conditions (Fig. 3D). Thus, Tyr phosphorylation is a trigger for rapid internalization of S1045Y-CFTR.
Inhibition of Tyr Phosphorylation Enhances Surface Levels of S1045Y-CFTR and Improves Its Function
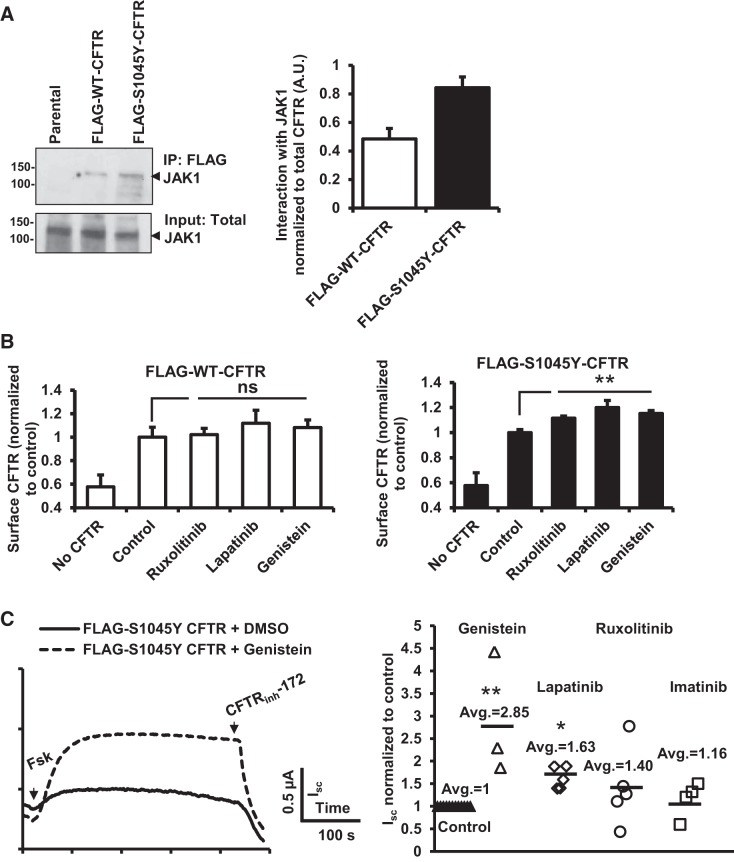
The pivotal component of the study would be determination of the specific tyrosine kinase that associates with the mutant protein. Through initial screening attempts with several tyrosine kinases, we narrowed it down to JAK1 as the tyrosine kinase binding partner of CFTR that preferentially associates with S1045Y-CFTR protein (Fig. 4A). Because Tyr phosphorylation of S1045Y-CFTR is the molecular trigger for its rapid internalization, we sought to determine whether inhibition of Tyr phosphorylation can prevent S1045Y-CFTR internalization. We used a surface-labeling assay to determine surface levels of CFTR. HEK 293 cells expressing WT- or S1045Y-CFTR were treated with the protein synthesis inhibitor CHX (100 μg/ml) for 3–4 h to allow selective study of the CFTR population present at the plasma membrane. Following CHX chase, cells were treated with various tyrosine kinase inhibitors as follows: 5 μM ruxolitinib (selective JAK1/2 inhibitor), 0.5 μM lapatinib (EGFR and ErbB2 inhibitor), or 50 μM genistein (generic protein tyrosine kinase inhibitor) for 60 min at 37°C with no-treatment controls in parallel. We observed that all of the tyrosine kinase inhibitors could marginally rescue the S1045Y-CFTR level but that there are no such effects observed for WT-CFTR (Fig. 4B). To functionally demonstrate the partial restoration of the S1045Y-associated defect, we mounted CFBEo− cells in Ussing chambers and studied CFTR chloride channel function for S1045Y-CFTR-expressing cells pretreated with tyrosine kinase inhibitors (50 μM genistein, 0.5 μM lapatinib, 0.5 μM imatinib, and 10 μM ruxolitinib) in response to forskolin (10 μM). We observed partial rescue (15–186% increase) of S1045Y-CFTR function most effectively with genistein (Fig. 4C). Because JAK1 inhibition could not completely rescue S1045Y-CFTR surface levels to WT-CFTR surface amounts, we predict that JAK1 is not the sole tyrosine kinase to preferentially interact with S1045Y-CFTR and that multiple kinases could be associated with the mutant protein.
Fig. 4.
Inhibition of tyrosine phosphorylation enhances surface levels of S1045Y-CFTR and improves its function. A: Western blot depicting the interaction of WT- and S1045Y-CFTR protein with Janus kinase 1 (JAK1). Bar graph on the right shows quantitation of interaction of JAK1 with CFTR. B: surface labeling assay to determine surface FLAG-WT-CFTR (left) and FLAG-S1045Y-CFTR (right) levels upon treatment with tyrosine kinase inhibitors as follows: ruxolitinib (5 μM), lapatinib (0.5 μM), or genistein (50 μM) following incubation with cyclohexamide (CHX). **P < 0.01. ns, Nonsignificant determined using Student's t-test for n = 3–4 experiments. C: left, representative traces of forskolin (Fsk)-stimulated S1045Y-CFTR-mediated Isc in CFBEo− cells following treatment with genistein (50 μM). Right, dot plot showing Fsk-stimulated Isc in response to various tyrosine kinase inhibitors quantified as normalized to untreated control S1045Y-CFTR-mediated currents. **P < 0.01 for genistein and *P < 0.05 for lapatinib determined using Student's t-test. No statistical significance could be established for imatinib and ruxolitinib treatment.
Tyr Phosphorylation in S1045Y-CFTR Promotes Docking of E3 Ubiquitin Ligase c-cbl on the Mutant Protein
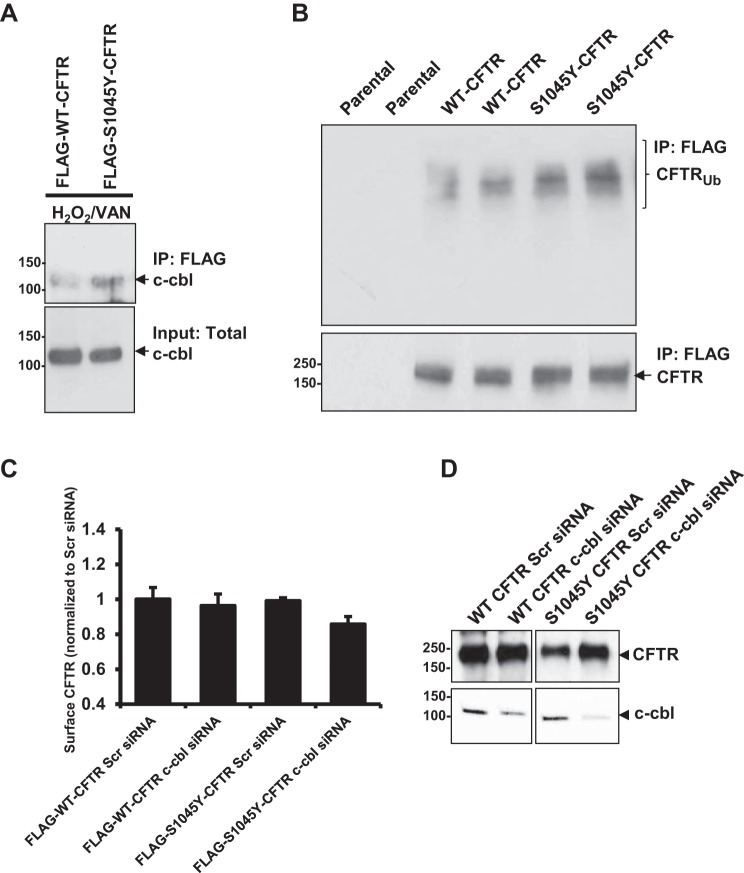
Because we hypothesized a gain of site of Tyr phosphorylation in the mutant protein, and subsequent binding of c-cbl, we tested whether S1045Y-CFTR would exhibit enhanced binding to c-cbl compared with WT-CFTR and whether a Tyr phosphorylation stimulus would enhance such binding. In our initial experiments, we observed a very weak and almost undetectable binding of c-cbl with S1045Y-CFTR. Therefore, we cross-linked the protein complexes to capture the interaction. FLAG-tagged WT- or S1045Y-CFTR proteins not subjected or subjected to H2O2/vanadate treatment for 60 min were cross-linked with the cell-permeable cross-linker dithiobis succinimidyl propionate (1 mM) at 37°C. We observed c-cbl coimmunoprecipitating with CFTR only under an oxidative condition, and S1045Y-CFTR exhibited increased binding with c-cbl compared with WT-CFTR (Fig. 5A). Because we established that c-cbl recognizes S1045Y-CFTR in its phosphorylated state, we next tested whether S1045Y-CFTR is more ubiquitinated than WT-CFTR. Western blot analysis using ubiquitin-specific antibody of immunoprecipitated WT- and S0145Y-CFTR proteins revealed that S1045Y-CFTR exhibits more ubiquitination compared with WT-CFTR (Fig. 5B). Next, we attempted to address the specificity of ubiquitination of S1045Y-CFTR through c-cbl. Toward this end, we knocked down c-cbl expression levels using c-cbl-directed Stealth RNAi siRNA. We then checked the surface levels of WT- and S1045Y-CFTR on downregulation of c-cbl expression using surface-labeling assay. Interestingly, we did not observe any rescue of the surface levels of S1045Y-CFTR with c-cbl downregulation and observed a slight decrease instead (Fig. 5C). These data suggest that the ubiquitinating event through c-cbl is not critical for S1045Y-CFTR endocytosis and that Tyr phosphorylation drives this process. A marginal decrease in the surface levels of S1045Y CFTR upon c-cbl knockdown is suggestive of the role of ubiquitination of the internalized protein pool in regulating the rate of internalization of the membrane protein. Based on these data, we predict that c-cbl-mediated ubiquitination would be important to promote lysosomal degradation of the mutant protein. To support this notion and establish c-cbl specificity toward ubiquitinating S1045Y-CFTR and facilitate its lysosomal recruitment, we evaluated total protein levels (cytoplasmic + membrane) and observed an increase in the total pool of S1045Y-CFTR upon c-cbl downregulation, with no such change observed in WT-CFTR amounts (Fig. 5D). Therefore, c-cbl-mediated ubiquitination regulates postinternalization fate of S1045Y-CFTR.
Fig. 5.
Tyrosine phosphorylation of S1045Y-CFTR promotes the docking of the E3 ubiquitin ligase c-cbl and ubiquitinates the mutant CFTR protein. A: Western blot depicts the interaction of WT- and S1045Y-CFTR proteins with c-cbl upon treatment with H2O2/vanadate. B: Western blot to test CFTR ubiquitination levels showing enhanced ubiquitination of S1045Y-CFTR protein compared with WT-CFTR protein. C: surface-labeling assay to determine the surface FLAG-WT-CFTR and FLAG-S1045Y-CFTR levels upon knockdown of c-cbl expression using Stealth siRNA. Scr siRNA, scrambled siRNA was used as a nonspecific knockdown control. D: Western blot to determine total WT and S1045Y CFTR levels with and without c-cbl knockdown.
DISCUSSION
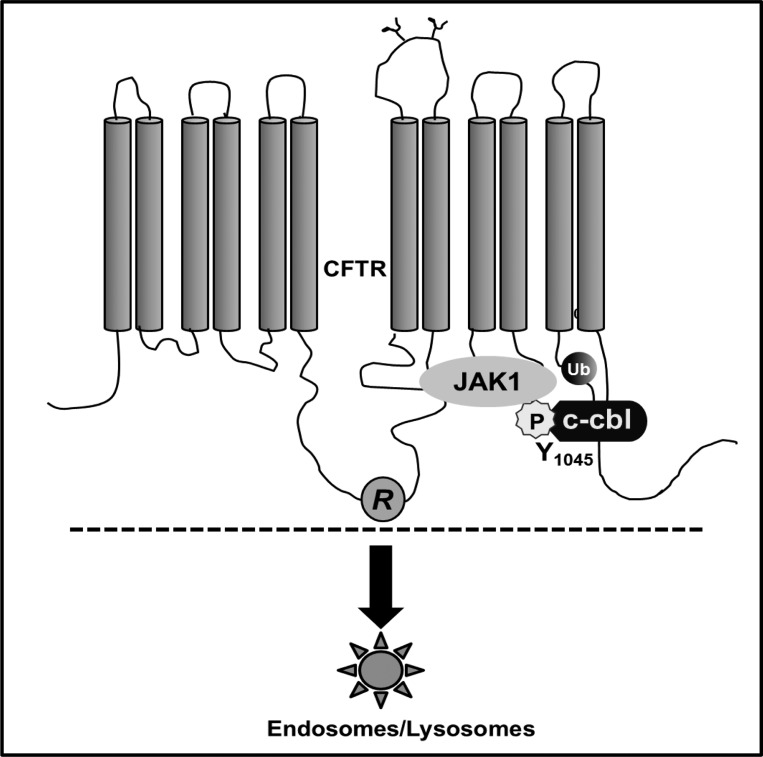
In this study, we elucidated the molecular basis of how the S1045Y mutation in CFTR can cause CF (Fig. 6). We demonstrated that 1) S1045Y-CFTR retains only 50% of normal CFTR activity in its homozygous form under a steady-state condition and hence, ∼25% when the heterozygote provided the other allele to encode a nonfunctional CFTR, such as in the case we present that the second mutation is a stop mutation. We would anticipate the patient to be on the borderline of 25% CFTR function, which explains his mild CF manifestations; 2) Tyr phosphorylation of the mutant protein is the molecular trigger for enhanced internalization of the protein; therefore, the state of Tyr phosphorylation in a given cell would determine the functional profile of the mutant protein and could be predicted to be lower than 50% of normal CFTR activity, and 3) the mutation causes the gain of an access site for ubiquitin ligase c-cbl to bind CFTR in its phosphorylated state at the mutated residue, resulting in an enhanced ubiquitination of the protein. This ubiquitination event facilitates the recruitment of the mutant protein into the lysosomes. There is a possibility, based on previous studies, that ubiquitination might accompany the mutant protein in the process of its lysosomal recruitment. Currently, we do not have evidence to demonstrate postendocytic events of S1045Y-CFTR mutant or whether ubiquitination of the protein is a postinternalization event. We speculate that the S1045Y-CFTR protein is monoubiquitinated rather than polyubiquitinated, since the latter would target the protein for proteosomal degradation, and we did not observe an increase in the levels of S1045Y-CFTR protein levels with MG-132, an inhibitor of proteosomal degradation (data not shown). Information on the fate of monoubiquitinated CFTR is currently limited. However, misfolding of CFTR within the cytosolic domains has been demonstrated to be a potent signal for attachment of ubiquitin chains and driving the protein into degradative pathways (14). We do not think that misfolding is a possibility for the enhanced ubiquitination of S1045Y-CFTR, since it exhibited levels of mature band C comparable to that of WT-CFTR. Consequently, S1045Y-CFTR is a strong candidate for a distinct monoubiquitination event.
Fig. 6.
Molecular machinery that assembles on S1045Y CFTR protein and causes it to loose surface retention and function by 50%.
Although a rare mutation, one individual homozygous for S1045Y-CFTR did have severe life-shortening lung involvement (20). This Galician female suffered from acute pancreatitis, allergic bronchopulmonary aspergillosis, and had chronic pulmonary symptoms (dyspnea, productive cough, nasal congestion, and diffuse bronchiectasis). She had clearly elevated sweat chloride levels (>90 Meq/l) and later acquired respiratory infections with Pseudomonas aeruginosa and Morganella morganii. Copious and thick mucus secretions were found at bronchoscopy, particularly in the upper lobe. The patient died with renal failure and progressive respiratory and hemodynamic deterioration. Our S1045Y CFTR carrier patient has so far had a milder CF disease course, but he is currently being followed for development of pulmonary complications. Because the residual function of S1045Y CFTR would vary depending on the oxidative state of the cell, it would be important to prevent oxidative stress in S1045Y CFTR patients. We identified JAK1 as a tyrosine kinase to demonstrate selective binding toward the mutant protein. Because we observed only a marginal improvement in S1045Y-CFTR function with a JAK1 inhibitor, there could be redundancy in Tyr phosphorylation of the mutant protein or additive action by several other tyrosine kinases. Genistein could act through dual mechanisms to benefit S1045Y-CFTR function by partially rescuing surface levels of S1045Y-CFTR and by potentiating CFTR function. Consequently, we observed more potent improvement in the function of S1045Y-CFTR in Isc measurements using genistein compared with other tyrosine kinase inhibitors. Soybeans and soy food (e.g., tofu, soy flour, soy milk) are enriched in isoflavones (genistein is an isoflavones derivative) and are consumed in significant amounts in Asian countries and also becoming popular in Western countries. Soy products are inexpensive and high in quality protein. Genistein constitutes 53% of total isoflavones in soy flour (3). Based on our in vitro data, we speculate that a soy diet and other isoflavones could benefit S1045Y patients by inhibiting Tyr phosphorylation, providing a unique personalized therapy for this rare CFTR mutation.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-080834 and DK-093045 to A. P. Naren and CF foundation grants to A. P. Naren and K. Arora (ARORA16F0).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.A. and A.P.N. conception and design of research; K.A., S.Y., E.B., C.L., and A.P.N. performed experiments; K.A., S.S., C.L., D.C.S., and A.P.N. analyzed data; K.A., S.Y., W.Z., C.M., C.L., and A.P.N. interpreted results of experiments; K.A. prepared figures; K.A. drafted manuscript; W.Z., C.M., E.B., D.C.S., and A.P.N. edited and revised manuscript; W.Z., S.S., C.L., D.C.S., and A.P.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank J. Denise Wetzel, Cincinnati Children's Hospital Medical Center Medical Writer, for editing the manuscript.
REFERENCES
- 1.Ameen N, Silvis M, Bradbury NA. Endocytic trafficking of CFTR in health and disease. J Cyst Fibros 6: 1–14, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson MP, Rich DP, Gregory RJ, Smith AE, Welsh MJ. Generation of cAMP-activated chloride currents by expression of CFTR. Science 251: 679–682, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Anupongsanugool E, Teekachunhatean S, Rojanasthien N, Pongsatha S, Sangdee C. Pharmacokinetics of isoflavones, daidzein and genistein, after ingestion of soy beverage compared with soy extract capsules in postmenopausal Thai women. BMC Clin Pharmacol 5: 2, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arora K, Moon C, Zhang W, Yarlagadda S, Penmatsa H, Ren A, Sinha C, Naren AP. Stabilizing rescued surface-localized deltaf508 CFTR by potentiation of its interaction with Na(+)/H(+) exchanger regulatory factor 1. Biochemistry 53: 4169–4179, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cihil KM, Zimnik A, Swiatecka-Urban A. c-Cbl reduces stability of rescued F508-CFTR in human airway epithelial cells: implications for cystic fibrosis treatment. Commun Integr Biol 6: e23094, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med 363: 2233–2247, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, Durie PR, Legrys VA, Massie J, Parad RB, Rock MJ, Campbell PW 3rd Cystic Fibrosis F. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr 153: S4–S14, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jehn BM, Dittert I, Beyer S, von der Mark K, Bielke W. c-Cbl binding and ubiquitin-dependent lysosomal degradation of membrane-associated Notch1. J Biol Chem 277: 8033–8040, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Krishnamurthy PC, Penmatsa H, Marrs KL, Wang XQ, Zaccolo M, Jalink K, Li M, Nelson DJ, Schuetz JD, Naren AP. Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell 131: 940–951, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lukacs GL, Chang XB, Bear C, Kartner N, Mohamed A, Riordan JR, Grinstein S. The delta F508 mutation decreases the stability of cystic fibrosis transmembrane conductance regulator in the plasma membrane. Determination of functional half-lives on transfected cells. J Biol Chem 268: 21592–21598, 1993. [PubMed] [Google Scholar]
- 11.Marmor MD, Yarden Y. Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene 23: 2057–2070, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Moon C, Zhang W, Ren A, Arora K, Sinha C, Yarlagadda S, Woodrooffe K, Schuetz JD, Valasani KR, de Jonge HR, Shanmukhappa SK, Shata MT, Buddington RK, Parthasarathi K, Naren AP. Compartmentalized accumulation of cAMP near complexes of multidrug resistance protein 4 (MRP4) and cystic fibrosis transmembrane conductance regulator (CFTR) contributes to drug-induced diarrhea. J Biol Chem 290: 11246–11257, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naren AP, Nelson DJ, Xie W, Jovov B, Pevsner J, Bennett MK, Benos DJ, Quick MW, Kirk KL. Regulation of CFTR chloride channels by syntaxin and Munc18 isoforms. Nature 390: 302–305, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Okiyoneda T, Barriere H, Bagdany M, Rabeh WM, Du K, Hohfeld J, Young JC, Lukacs GL. Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science 329: 805–810, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okiyoneda T, Lukacs GL. Fixing cystic fibrosis by correcting CFTR domain assembly. J Cell Biol 199: 199–204, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penmatsa H, Zhang W, Yarlagadda S, Li C, Conoley VG, Yue J, Bahouth SW, Buddington RK, Zhang G, Nelson DJ, Sonecha MD, Manganiello V, Wine JJ, Naren AP. Compartmentalized cyclic adenosine 3′,5′-monophosphate at the plasma membrane clusters PDE3A and cystic fibrosis transmembrane conductance regulator into microdomains. Mol Biol Cell 21: 1097–1110, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piper RC, Dikic I, Lukacs GL. Ubiquitin-dependent sorting in endocytosis. Cold Spring Harbor Perspect Biol 6, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prince LS, Peter K, Hatton SR, Zaliauskiene L, Cotlin LF, Clancy JP, Marchase RB, Collawn JF. Efficient endocytosis of the cystic fibrosis transmembrane conductance regulator requires a tyrosine-based signal. J Biol Chem 274: 3602–3609, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Rabeh WM, Bossard F, Xu H, Okiyoneda T, Bagdany M, Mulvihill CM, Du K, di Bernardo S, Liu Y, Konermann L, Roldan A, Lukacs GL. Correction of both NBD1 energetics and domain interface is required to restore DeltaF508 CFTR folding and function. Cell 148: 150–163, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rana-Diez P, Colon C, Alonso-Fernandez JR, Solar A, Barros-Tizon JC, Barros-Casas D, Sirvent J, Carracedo A, Barros F. Three novel mutations in the CFTR gene identified in Galician patients. J Cyst Fibros 7: 520–522, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Robinson M, Bye PT. Mucociliary clearance in cystic fibrosis. Pediatr Pulmonol 33: 293–306, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Rowntree RK, Harris A. The phenotypic consequences of CFTR mutations. Ann Hum Genet 67: 471–485, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt MH, Furnari FB, Cavenee WK, Bogler O. Epidermal growth factor receptor signaling intensity determines intracellular protein interactions, ubiquitination, and internalization. Proc Natl Acad Sci USA 100: 6505–6510, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma M, Pampinella F, Nemes C, Benharouga M, So J, Du K, Bache KG, Papsin B, Zerangue N, Stenmark H, Lukacs GL. Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes. J Cell Biol 164: 923–933, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med 372: 351–362, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wine JJ, Joo NS. Submucosal glands and airway defense. Proc Am Thoracic Soc 1: 47–53, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Ye S, Cihil K, Stolz DB, Pilewski JM, Stanton BA, Swiatecka-Urban A. c-Cbl facilitates endocytosis and lysosomal degradation of cystic fibrosis transmembrane conductance regulator in human airway epithelial cells. J Biol Chem 285: 27008–27018, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zielenski J. Genotype and phenotype in cystic fibrosis. Respiration 67: 117–133, 2000. [DOI] [PubMed] [Google Scholar]