Abstract
Baculoviruses are commonly used for recombinant protein and vaccine production. Baculoviruses are nonpathogenic to vertebrates, have a large packaging capacity, display broad host and cell type tropism, infect both dividing and nondividing cells, and do not elicit strong immune or allergic responses in vivo. Hence, their use as gene delivery vehicles has become increasingly popular in recent years. Moreover, baculovirus vectors carrying mammalian regulatory elements can efficiently transduce and express transgenes in mammalian cells. Based on the finding that heparan sulfate, which is structurally similar to heparin, is an attachment receptor for baculovirus, we developed a novel scalable baculovirus purification method using heparin-affinity chromatography. Baculovirus supernatants were loaded onto a POROS heparin column, washed to remove unbound materials, and eluted with 1.5 mol/l NaCl, which yielded a recovery of purified baculovirus of 85%. After ultracentrifugation, baculovirus titers increased from 200- to 700-fold with overall yields of 26–29%. We further show that baculovirus particles were infectious, normal in morphology and size, despite high-salt elution and shear forces used during purification and concentration. Our chromatography-based purification method is scalable and, together with ultracentrifugation and/or tangential flow filtration, will be suitable for large-scale manufacturing of baculovirus stocks for protein and vaccine production and in gene therapy applications.
Introduction
The baculovirus vector system was originally developed for the production of high levels of recombinant proteins and vaccines using insect cell cultures.1–3 However, several studies have shown that baculovirus can infect mammalian cells but is unable to replicate due to transcriptional silencing of most of the baculoviral genes. Baculovirus vectors carrying mammalian regulatory elements, known as bacmams, can efficiently transduce and express transgenes in mammalian cells both in vitro and in vivo.4,5 These properties make baculovirus vectors promising candidates for gene therapy application.
Baculoviruses have several distinct advantages as a gene transfer vector over other nonintegrating viral vectors such as adenovirus and adeno-associated virus (AAV). Baculovirus is nonpathogenic to vertebrates; has a large packaging capacity (up to 38 kb), has a broad host and tissue tropism, infects both dividing and nondividing cells, and does not elicit strong immune and allergic responses in infected hosts.6 Since baculovirus does not integrate into the genome, there is no significant risk of insertional mutagenesis leading to aberrant transformation. Moreover, baculovirus is easy to propagate in large-scale in serum-free suspension culture.7–9 Therefore, recombinant baculovirus vectors are attractive as novel gene therapy tools for transient and high-level transgene expression.5,7,8,10
The prototype baculovirus Autophaga californica multicapsid nucleopolyhedrovirus (AcMNPV) contains a circular double stranded genome of 134 kb with 154 open reading frames. The virion particle has a rod-shaped nucleocapsid (25 × 260 nm) which is surrounded by envelope glycoproteins.11
Baculoviruses infect a broad range of cell types including most of the mammalian cell lines, primary cells, adult, and embryonic stem cells.12–14 Several studies have revealed that the central nervous system, liver, eye, ovary, prostate, and testis can serve as targets for baculovirus-mediated gene delivery.8,15 Preclinical studies of cancer gene therapy have successfully been conducted in animal models with baculoviral vectors containing therapeutic genes, such as suicide genes, tumor suppressor genes, and genes encoding tumor-specific antigens.16,17
When introducing transgenes in vivo, it is important that baculovirus particles are pure with minimum levels of producer cell- and culture media-derived impurities to avoid toxicity, inflammation, and an immune response. In addition, purification methods should be scalable so that they can be used for large-scale manufacturing.2,3,18 Chromatography-based virus purification methods yield high purity, are easy to scale-up, and can be conducted using a closed system compatible with current good manufacturing practices. In recent years, various chromatography procedures have been successfully applied to large-scale purification of a variety of viral vectors including AAV, adenovirus, retrovirus, baculovirus, and virus-like particles.18–20
Heparan sulfate has been described as a receptor for attachment of baculovirus particles to mammalian cells.21,22 The heparin-binding domains of the baculovirus envelope are responsible for receptor-ligand interaction. Here, we demonstrate that baculovirus vector particles can be purified using scalable heparin affinity column chromatography and can be effectively concentrated by ultracentrifugation without loss of infectivity.
Results
Production of baculovirus
Baculovirus supernatants were produced by transient transfection of bacmid DNA into Sf9 cells in an adherent culture, and transfection efficiency was evaluated using a fluorescence microscope. Two days post-transfection, GFP expression was detected in transfected cells by fluorescence microscopy (data not shown). Since the baculovirus vector is replication competent, GFP expression gradually increased in Sf9 cell population due to infection of new cells with newly produced baculovirus particles secreted into the growth medium. Baculovirus supernatants were harvested 5 days after transfection when almost all of the cells showed GFP expression. This baculovirus stock was designated as passage 1 (P1) stock. The P1 stock was used to infect newly seeded adherent culture of Sf9 cells. About 4–5 days after infection, almost all of the cells showed GFP expression. The supernatant was harvested and was designated as P2 stock, which was subsequently used to infect newly seeded Sf9 cells in suspension in an Erlenmeyer flask. Baculovirus infection in the Sf9 suspension culture was monitored by GFP expression using a flow cytometer (Figure 1a). As the infection progressed, the percentage of GFP-expressing cells gradually increased. At the end of the infection cycle, most of the cells showed cytotoxicity with an increased cellular diameter, which was a sign of high baculovirus infection. Baculovirus-infected Sf9 cells were also stained with anti-gp64 antibody. More than 98% of the cells expressed baculoviral gp64 envelope glycoprotein as well as GFP (Figure 1b). Baculovirus cultures were harvested and supernatant was separated from the cells by low-speed centrifugation. This material was designated as P3 stock, which was further expanded in Sf9 cells until the desired volume was obtained. Supernatants were treated with Benzonase nuclease at 50 Units/ml prior to chromatography to reduce viscosity. Treating baculovirus supernatant with Benzonase nuclease did not affect the baculovirus titer (data not shown). Supernatants were passed through a 0.45-micron filter and used in subsequent experiments or stored in the dark at 4 °C in the presence of 0.5% bovine serum albumin (BSA).
Figure 1.
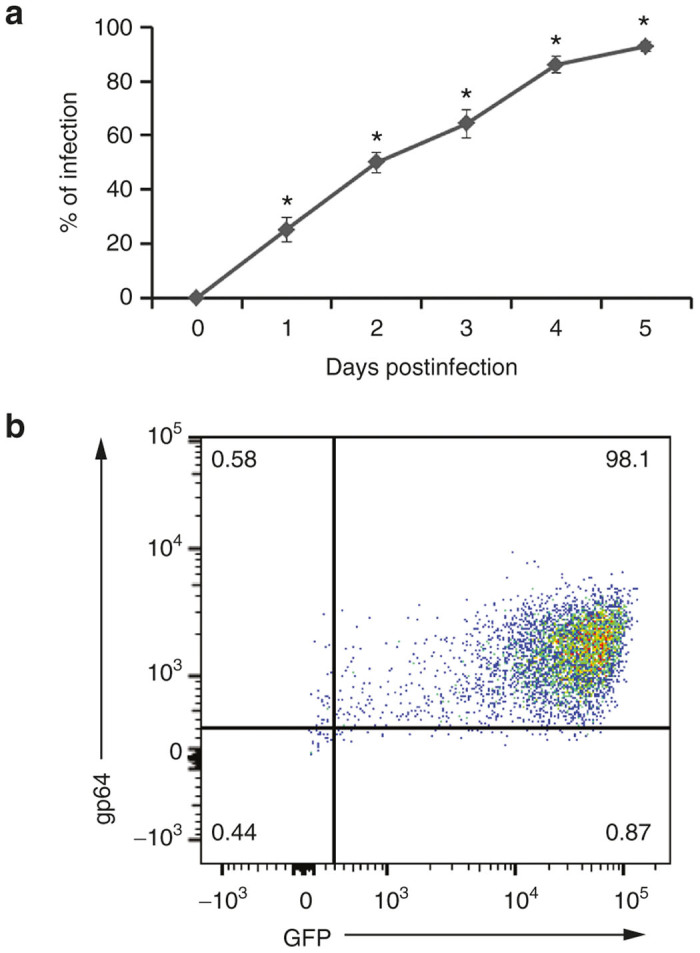
Propagation of baculovirus in Sf9 cells. (a) Sf9 cells were infected with baculovirus supernatant. The progress of baculovirus infection was monitored by green fluorescent protein (GFP) expression. The values represent the mean ± standard error of the mean (SEM) of triplicate experiments (*P ≤ 0.05, as compared to 0 hours of baculovirus infection). (b) Baculovirus infected Sf9 cells were stained with anti-gp64 antibody and were analyzed with a flow cytometer.
Heparin inhibited baculovirus infection
Heparan sulfate, which is structurally similar to heparin, has been identified as a receptor for baculovirus.21,22 We first tested whether heparin could bind and inhibit baculovirus infection of mammalian cells. Increasing concentrations of heparin from 0.5 µg/ml to 10.0 µg/ml were added to baculovirus supernatants, incubated for 1 hour at room temperature, and added to HT1080 cells at a multiplicity of infection of 1 and 2 in separate experiments. One hour after infection, cells were thoroughly washed with phosphate-buffered saline (PBS) to remove unbound baculovirus and heparin. Cells were subsequently cultured in the presence of growth media for another 2 days and analyzed for infection as shown by the expression of GFP using a flow cytometer. We found that heparin inhibited baculovirus infection in a dose-dependent manner (Figure 2). More than 90% of baculovirus infection was inhibited with as low as 5 µg/ml heparin. A similar level of inhibition was detected at a multiplicity of infection of 1 (Figure 2a) and 2 (Figure 2b). Thus, inhibition of baculovirus infection by heparin confirms our previously published data that baculovirus binds heparan sulfate to enter cells,22 and suggested that heparin columns could likely be used for purification.
Figure 2.
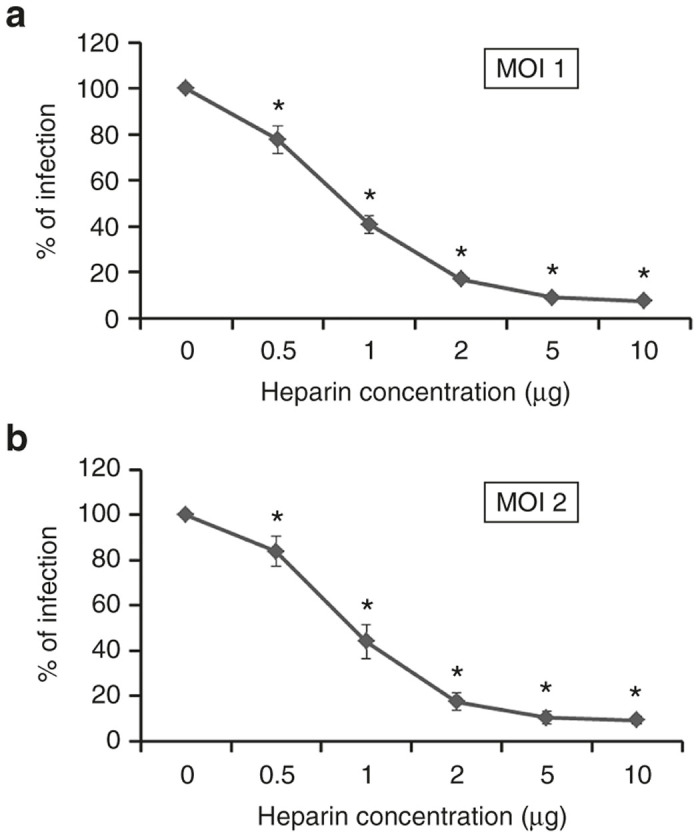
Inhibition of baculovirus infection with heparin. Baculovirus was incubated with the indicated concentrations of heparin for 1 hour prior to a 1-hour adsorption of the baculovirus-heparin mixture to HT1080 cells for infection. GFP expression was analyzed 48 hours post-infection using a flow cytometer. (a) Baculovirus was used at an multiplicity of infection (MOI) of 1. (b) Baculovirus was used at MOI of 2. Each point represents the average percentage decrease in GFP expression. The values represent the mean ± standard error of the mean (SEM) of triplicate experiments (*P ≤ 0.05, when compared to 0 µg/ml of heparin).
Stability of baculovirus
Before designing a purification strategy for baculovirus, we tested the stability of infectious baculovirus particles under various buffer, pH, and salt conditions. First, we tested the effect of three buffers at neutral pH, including 20 mmol/l Tris–HCl (hydroxymethyl aminomethane-hydrochloric acid), 20 mmol/l sodium phosphate, and PBS. All buffers contained 150 mmol/l NaCl and were adjusted to pH 7.0. Baculovirus supernatant was incubated individually in each of the buffers for up to 6 hours and tested for infectivity on HT1080 cells. We found that the stability of baculovirus in all three buffers was similar (Figure 3a). However, baculovirus infections were significantly reduced with time in all of the buffer systems when compared to the initial infection at 0 hour (P ≤ 0.05). Hence, purification of baculovirus needs to be carried out rapidly to retain maximal infectivity postpurification.
Figure 3.
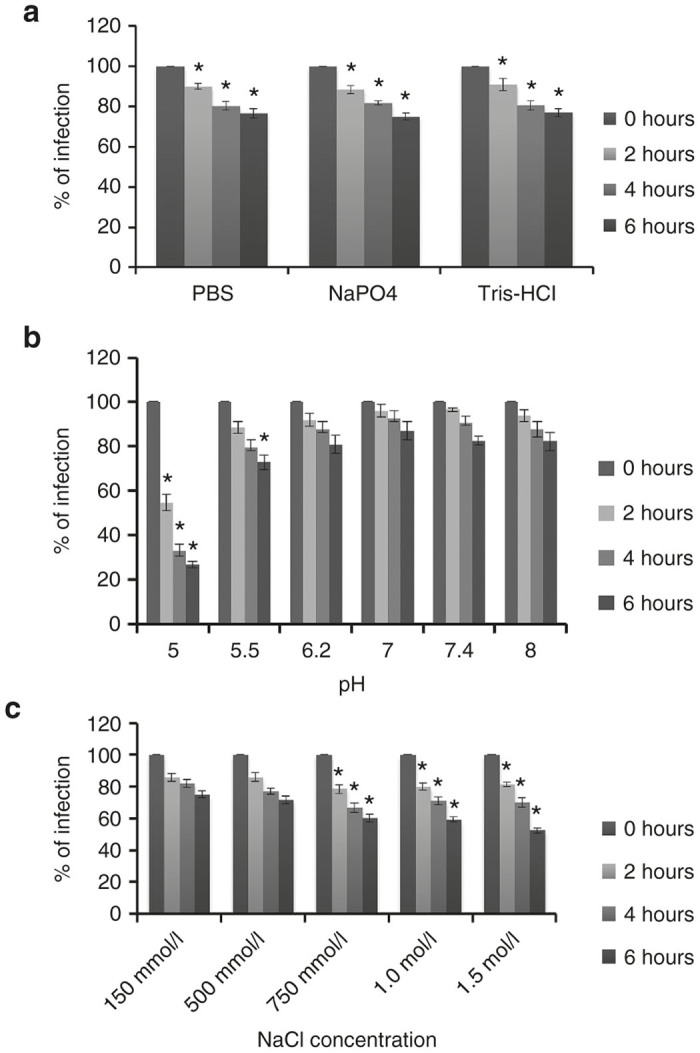
Baculovirus stability testing. Baculovirus was incubated in each of the indicated buffers for up to 6 hours and used to infect HT1080 cells. GFP fluorescent expression was analyzed 48 hours post-infection using a flow cytometer. The values represent the mean ± standard error of the mean (SEM) of triplicate experiments (a) Baculovirus was incubated in three different buffer systems containing 150 mmol/l NaCl (*P ≤ 0.05, when compared to the 0 hour of incubation of baculovirus in the corresponding buffer). (b) Baculovirus was incubated in sodium phosphate buffer containing variable pH (*P ≤ 0.05, when compared with the incubation of baculovirus in buffer with neutral pH). (c) Baculovirus was incubated in sodium phosphate buffer with increasing concentration of NaCl (*P ≤ 0.05, when compared to incubation of baculovirus in isotonic buffer, 150 mmol/l NaCl).
Next, we tested baculovirus stability after incubation in sodium phosphate buffers with a pH ranging from 5 to 8 for up to 6 hours at room temperature. Baculovirus was stable in pH from 6.2 to 8.0 without any significant loss of infectivity when compared with a buffer containing neutral pH (Figure 3b). However, baculovirus significantly became inactivated and lost its ability to infect cells after 6 hours at pH 5.5 and within 2 hours of incubation at pH 5.0 (Figure 3b). It is known that the major envelope glycoprotein of baculovirus, gp64, binds heparin in a pH-dependent manner, with a stronger binding at pH 6.2 than at 7.4.26 Therefore, the loading of baculovirus onto the heparin column should preferably be carried out at pH 6.2 while elution can be performed at pH 8.0.
Next, we tested the stability of infectious baculovirus after exposure to an increasing concentration of NaCl, ranging from 150 mmol/l to 1.5 mol/l (Figure 3c). Our data showed that infectious baculovirus could tolerate exposure to 500 mmol/l NaCl for up to 6 hours without significant loss of infectivity when compared with isotonic buffer. However, baculovirus showed significant infection inactivation in buffer containing 750 mmol/l or more NaCl. The higher the salt concentration in the buffer, the more rapid inactivation of infectivity was observed. Also, the biological inactivation of baculovirus in the presence of high concentration of salt was irreversible. This was tested by reducing the NaCl concentration after exposure (data not shown). Therefore, the time that baculovirus is exposed to high salt containing buffer during purification needs to be limited.
Purification and concentration of baculovirus
Based on the finding that baculovirus infection of HT1080 cells is inhibited by heparin, and the report that baculovirus binds to cell surface heparan sulfate,21,22 we tested whether baculovirus could be purified using heparin affinity chromatography. Prior to chromatography, baculovirus supernatants were treated with 50 Units/ml of Benzonase nuclease for 2 hours at room temperature. The supernate was subsequently centrifuged and filtered through a 0.45-micron filter to remove coarse cellular debris and aggregates. Filtered baculovirus supernatants were loaded onto a POROS 50 µm heparin affinity chromatography column. We observed that our 7.9 ml heparin column became gradually saturated during loading with 250–280 ml of supernatant containing baculovirus particles and protein contaminants. This was evident by the presence of infectious baculovirus in the flow-through at the end of the chromatography run (data not shown). The heparin column was washed with sodium phosphate buffer containing 150 mmol/l NaCl (pH 7.0) until the ultraviolet (UV) absorbance curve (280 nm) returned to the baseline and became stabilized, demonstrating removal of unbound material. We did not observe any significant loss of baculovirus particles in the flow-through during washing the column. Initially, the heparin bound baculovirus particles were eluted with buffers containing 150 mmol/l and 1.5 mol/l NaCl (pH 8.0) using gradient elution. Individual chromatography fractions were used to infect HT1080 cells (data not shown). The optimal NaCl concentration for elution was estimated based on the conductivity and highest peak of eluted fraction. After several trial runs, we observed that baculovirus particles could be eluted with 1.5 mol/l of NaCl at pH 8.0. A sharp peak was observed during elution which corresponded to the major baculovirus fraction (Figure 4). The optimized conditions for sample loading, washing, and elution described here yielded 54–85% recovery of baculovirus (n = 5). The eluted fractions were further concentrated ~200- to 700-fold with ultracentrifugation and formulated in PBS containing 0.5% bovine serum albumin which yielded a 26–29% recovery (n = 3). Table 1 describes the recovery of purified and concentrated baculovirus samples.
Figure 4.
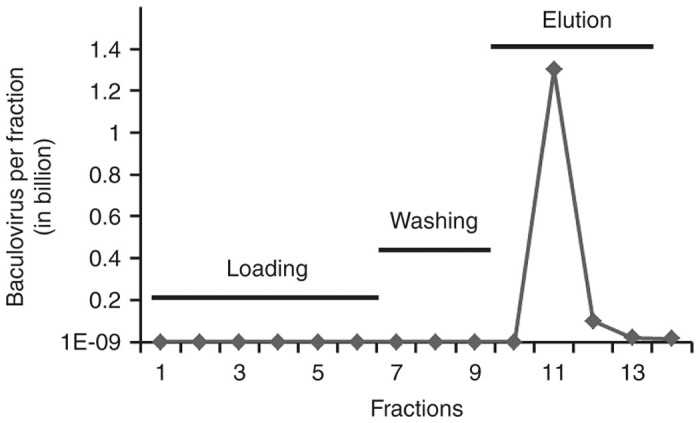
Purification of baculovirus supernatants with heparin affinity chromatography. Baculovirus sample was purified using a POROS heparin column with optimal conditions for sample loading and washing as described. Virus was eluted with 1.5 mol/l NaCl and diluted with phosphate-buffered saline (PBS). Baculovirus infectivity was estimated by infection of HT1080 cells with the diluted fractions of baculovirus. The line with dark diamonds shows the total infectious units (IU) of baculovirus in billions in each fraction.
Table 1. Estimated recovery of baculovirus samples after purification and concentration.
|
Recovery of baculovirus (%)a |
|||||
|---|---|---|---|---|---|
| Experiment number | Volume (ml) | Chromatography | Ultracentrifuge | Final fold-concentration | Final titers (IU/ml)b |
| 1 | 150 | 65.0 | NDc | NDc | – |
| 2 | 150 | 77.0 | NDc | NDc | – |
| 3 | 250 | 54.2 | 29.1 | 278 | 1.2 × 109 |
| 4 | 280 | 85.5 | 25.7 | 233 | 1.1 × 109 |
| 5 | 270 | 84.9 | 26.1 | 675 | 4.0 × 108 |
HT1080 cells were used to estimate the recovery of infectious baculovirus.
IU/ml: Infectious Units per milliliter.
ND, not done.
Purity and identity of purified baculovirus
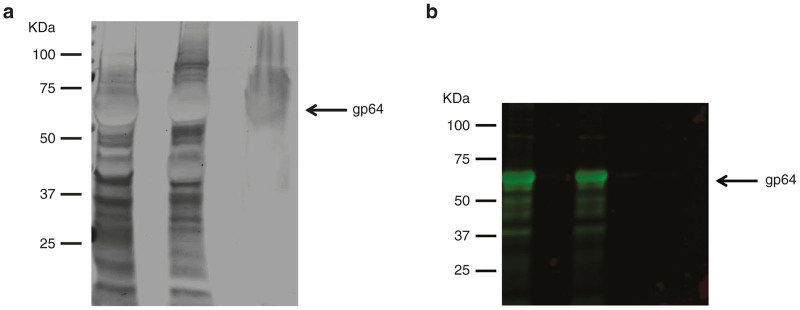
The purity and identity of baculovirus particles were evaluated by silver staining and immunoblotting in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) gels. Concentrated crude baculovirus (left lane), heparin chromatography-purified baculovirus (middle lane), and chromatography flow-through (right lane) showed the protein bands by silver staining (Figure 5a). The flow-through contained protein bands from cellular debris and proteins of culture medium that did not bind to the column during sample loading. As expected, the purified sample showed some protein contaminants that copurified with viral particles by binding to heparin. The 64 KDa protein band was predicted as the baculoviral gp64 envelope glycoproteins. Immunoblotting analysis with anti-gp64 antibody detected the baculoviral gp64 protein in crude (left lane) and purified (middle lane) samples (Figure 5b). Although silver staining revealed protein bands in the flow-through sample (Figure 5a, right lane), immunoblotting could not detect any significant band (Figure 5b, right lane).
Figure 5.
Purity and identity of purified baculovirus. Baculovirus particles were evaluated for (a) purity by silver staining of sodium dodecyl sulfate (SDS)– polyacrylamide gel electrophoresis (PAGE) gel, and (b) identity by immunoblotting using anti-gp64 antibody specific for baculoviral envelope glycoprotein. Left lane, concentrated crude baculovirus; Middle lane, heparin chromatography-purified baculovirus; and right lane, flow-through of chromatography run.
Purified and concentrated baculovirus particles were visualized by transmission electron microscopy which showed rod-shaped baculovirus particles 300–400 nm in length with some aggregates (Figure 6a). Purified and concentrated baculovirus supernatant was diluted to transduce HT1080 cells. Two days after transduction, GFP expression was detected (Figure 6b). Together these data demonstrated that heparin-affinity chromatography and ultracentrifugation can be used to effectively capture, purify and concentrate baculovirus vectors without affecting the size and morphology of the baculovirus particles. The data also demonstrated that baculovirus particles were infectious after purification.
Figure 6.
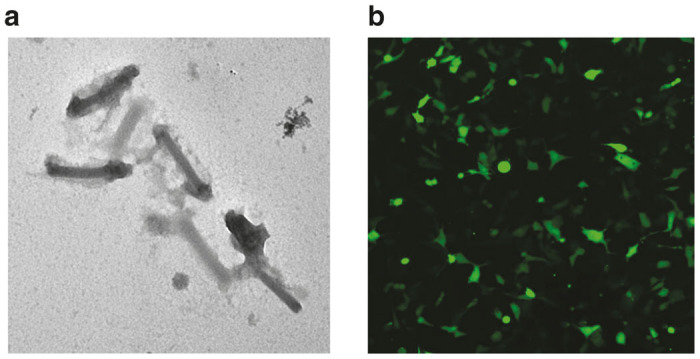
Morphology of purified baculovirus and Green flourescence protein (GFP) expression in cells. (a) Transmission electron micrograph of purified and concentrated recombinant baculovirus particles. (b) Purified baculovirus was transduced into HT1080 cells and GFP expression was detected 2 days after transduction with a fluorescence microscope.
Discussion
In this study, we have optimized each of the purification parameters to maximize yield and to prevent inactivation of infectious baculovirus particles (Figure 7). Wang and colleagues previously tried to purify baculovirus using heparin affinity chromatography. However, in contrast to the 85% recovery obtained here, they only recovered 2% of the baculovirus particles from the heparin column with low-salt elution buffer.27 Herein, we show that most of the heparin-bound infectious baculovirus particles can be eluted using high salt elution buffer if diluted immediately with PBS to prevent inactivation by hyperosmotic shock.
Figure 7.
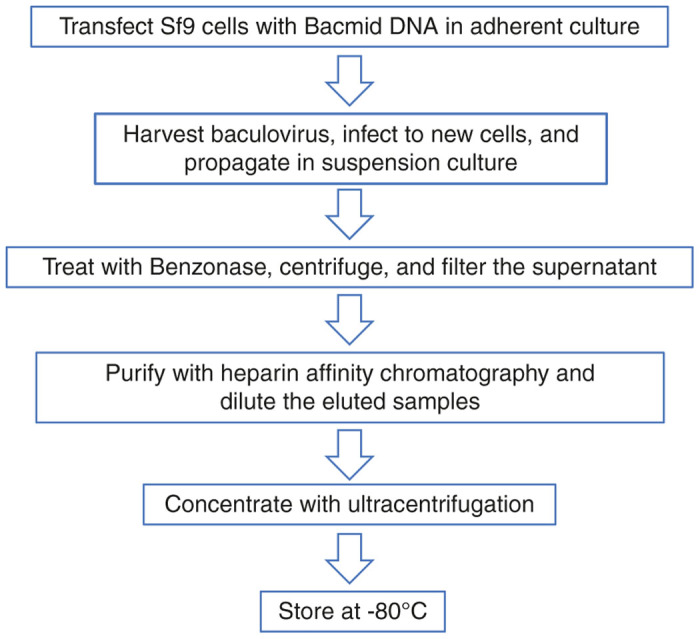
Flow diagram of baculovirus production and purification. Sf9 producer cells were seeded in cell culture-treated vessels and bacmid DNA was transfected into the cells. Baculovirus supernatants were harvested and transferred to uninfected Sf9 cells in adherent culture, and subsequently propagated in suspension culture. Baculovirus supernatant was treated with Benzonase nuclease, centrifuged, and filtered. Finally, baculovirus was purified by heparin affinity chromatography, diluted with phosphate buffered saline (PBS), and concentrated aseptically by ultracentrifugation. Baculovirus supernatants were stored at −80°C.
Concentrating virus with ultracentrifugation without chromatography purification results in precipitation of virus particles, cellular debris, and contaminating cellular proteins,28 which can be toxic for in vivo applications and may contribute to immune and allergic responses. In this study, we have developed a scalable baculovirus purification method using heparin affinity column chromatography that is compatible with large-scale good manufacturing practices production. We subsequently concentrated our baculovirus samples using ultracentrifugation, which is not linearly scalable in a large-scale process. An alternative approach for large-scale production may be the application of tangential flow filtration.29,30 Tangential flow filtration not only allows concentration of the product, it simultaneously removes small molecule contaminants during processing of the samples and allows for buffer exchange.
Baculovirus producer cells lyse during baculovirus production due to cytopathic effect of baculovirus infection,3 which results in cellular genomic DNA and RNA contamination of the supernatants. This, in turn, increases the viscosity of supernatant which interferes with the downstream purification. This was resolved by the addition of Benzonase nuclease prior to purification which digests both DNA and RNA over a wide range of temperatures and pH.31 We observed that Benzonase nuclease can be safely used in the purification of baculovirus without loss of infectious titer.
Baculovirus particles bud from the producer cells and are known to form aggregates at high titers and at low pH during insect cell culture.32 Aggregation of baculovirus reduces the titer after purification. pH is a key parameter that affects stability and aggregation of virus particles. In general, acidic pH causes loss of baculovirus infectivity and increases the aggregation of baculovirus particles. Previous studies have shown that poliovirus and reovirus aggregate in clumps of up to several hundred particles when diluted in low pH solution.33 During purification, we have eluted at pH 8.0, to avoid acidic pH induced aggregation of baculovirus in the down-stream processing.
We also demonstrate that if baculovirus is exposed to high-salt buffer for a prolonged period of time, baculovirus particles are inactivated irreversibly. Hence, elution followed by rapid dilution to near iso-osmolar conditions, as demonstrated here, retains infectivity.
We show that baculovirus particles strongly bind to heparin affinity media based on their affinity for heparan sulfate.21 This effectively separates viral particles from small cellular debris, which does not bind and ends up in the flow-through during sample loading and during washing with low salt buffer. The baculovirus-heparin interaction appears stable but reversible which requires a high-salt buffer for dissociation, as demonstrated in our study.
POROS heparin can capture a sample at speeds of 1,000–5,000 cm/hour when compared to the 50–360 cm/hour speed needed for conventional heparin medium columns. POROS heparin media have two discreet classes of pores. Large “through pores” allow convection flow to occur through the particles themselves, quickly carrying sample molecules to short “diffusive” pores inside. By reducing the distance over which diffusion needs to occur, the time required for sample molecules to interact with interior binding sites is also reduced, yielding better performance than the other heparin media.34,35 We carefully optimized binding conditions and found POROS heparin to be superior in its ability to capture baculovirus particles when compared with Hi-Trap or Capto-Heparin (not shown). The ability to be able to bind baculovirus at higher sample speeds and more effectively with POROS heparin is important to minimize downstream processing time in large-scale manufacturing.
Electron microscopy showed that most of the baculovirus particles had a normal morphology and size despite the exposure to high-salt and strong shear forces during purification. It is unclear whether the low level of aggregation observed after ultracentrifugation is an artifact caused by the pelleting and inadequate resuspension of the pellet, or was simply the result of the high concentration of virus particles. Irrespective, the purified baculovirus particles were infectious and were able to express the transgene in the target cells.
In conclusion, we have developed a novel affinity chromatography method to purify baculovirus particles which selectively bind to immobilized heparin. The baculovirus purification process described here paves the way to develop a scalable method which can be used at industrial scale for production of gene therapy vectors, recombinant proteins, and vaccines.
Materials and Methods
Cell culture
The Spodoptera frugiperda, Sf9 insect cells (Invitrogen, Carlsbad, CA) were cultured in a MaxQ 8000 (Thomas Scientific, Swedesboro, NJ) orbital shaker incubator at 28°C in polycarbonate Erlenmeyer flasks (Corning, Corning, NY) containing serum-free HyClone SFX-insect culture media (HyClone, Logan, UT). The human fibrosarcoma cell line, HT1080 was grown in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) supplemented with 10% fetal bovine serum (FBS), 2 mmol/l GlutaMax, 1 mmol/l sodium pyruvate, 1% nonessential amino acids, and 1% penicillin-streptomycin.23,24
Production of baculovirus
Baculovirus donor plasmid (pFBGR) containing green fluorescent protein (GFP)25 was kindly provided by Robert Kotin (National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD). The GFP expression of this vector is driven by the cytomegalovirus promoter in mammalian cells and by a baculovirus promoter in insect cells. Recombinant baculovirus plasmids (bacmids) were constructed using the Bac-to-Bac Baculovirus Expression System (Invitrogen). This system uses site-specific transposon elements to insert donor vector sequences into AcMNPV-derived bacmid DNA which is maintained in Escherichia coli strain DH10Bac (Invitrogen). Recombinant bacmid DNA was purified from a bacterial colony which was grown in Luria-Bertani (LB) broth containing ampicillin, kanamycin, and tetracycline. The Sf9 cells were seeded in a six-well tissue culture-treated dish and allowed to attach for 1 hour at 28ºC. The bacmid DNA was transfected into Sf9 cells using Cellfectin II (Life Technologies, Grand Island, NY) in serum free unsupplemented Grace insect culture media (Invitrogen). Five hours after transfection, cells were washed and cultured with HyClone SFX-insect culture media for 5 days at 28 °C. Baculovirus supernatants were harvested when all of the producer cells were infected and showed cytopathic effects.
Baculovirus was amplified by infecting the Sf9 cells in suspension culture in an orbital shaker incubator at 28 °C in polycarbonate Erlenmeyer flasks containing serum-free HyClone SFX-insect culture media. About 2–3 days after infection, almost all producer cells showed a cytopathic effect, which is an indication of baculovirus production. Baculovirus-containing supernatants were harvested and treated with 50 units/ml Benzonase nuclease (Sigma) to digest the genomic DNA and RNA. Supernatants were filtered and stored with 0.5% bovine serum albumin protected from light at 4ºC for short-term (up to 90 days) or at –80ºC for long-term storage.
Heparin-binding assay
Baculovirus supernatants were diluted in 1 ml of DMEM containing 10% FBS. Heparin (Sigma, St. Louis, MO) was added to the diluted virus at 0, 0.5, 1.0, 2.0, 5.0, and 10.0 µg/ml. The heparin-baculovirus reaction mixture was incubated at room temperature for 1 hour and added to HT1080 cells seeded at least 18 hours before infection. After 1 hour, cells were washed thoroughly using PBS to remove unbound baculovirus and heparin. The cells were cultured for another 48 hours and analyzed for baculovirus infection (as indicated by GFP expression) using the LSR Fortessa flow cytometer (BD Biosciences, San Jose, CA).
Stability testing of baculovirus
Baculovirus was tested for stability in several buffers, including 20 mmol/l Tris–HCl, 150 mmol/l NaCl (pH 7.0), PBS (pH 7.0), and 20 mmol/l sodium phosphate, 150 mmol/l NaCl (pH 7.0). Baculovirus supernatants were diluted in all three buffers individually and incubated for 0, 2, 4, or 6 hours at room temperature. HT1080 cells were infected with the diluted baculovirus supernatants and incubated for 1 hour. Cells were washed thoroughly with PBS to remove unbound baculovirus particles, cultured for another 2 days, and analyzed for baculovirus infection (GFP expression) using a flow cytometer.
The pH stability of baculovirus was tested in 20 mmol/l sodium phosphate buffer containing 150 mmol/l of NaCl. The pH of the sodium phosphate buffer was adjusted to 5.0, 5.5, 6.2, 7.0, 7.5, or 8.0. Baculovirus was diluted in each of the pH adjusted sodium phosphate buffers and incubated for 0, 2, 4, and 6 hours at room temperature. The diluted virus was used to infect HT1080 cells and incubated for 1 hour. Cells were washed thoroughly with PBS, cultured for another 2 days, and analyzed for baculovirus infection as described above.
The stability of baculovirus was also tested in the presence of different concentrations of NaCl. Baculovirus was diluted in 20 mmol/l sodium phosphate (pH 7.0) containing 150, 500 , 750 , 1.0 , or 1.5 mol/l NaCl and incubated for 0, 2, 4, and 6 hours at room temperature. HT1080 cells were exposed to diluted baculovirus, incubated for 1 hour, washed, and analyzed as described above.
Purification and concentration of baculovirus
Baculovirus supernatants were purified using a POROS Heparin 50 µm column (10 × 10 cm, 7.9 ml) (Invitrogen) on an AKTA Avant 150 chromatography system equipped with Unicorn software (GE Healthcare, Pittsburgh, PA). The column was equilibrated with five column volumes (CV) of sodium phosphate buffer (pH 7.0) containing 150 mmol/l NaCl. Baculovirus supernatants were loaded onto the heparin column using the sample pump on the AKTA Avant system at a linear flow rate of 150 cm/hr and a 4 minutes residence time. The column was washed with 10 CV of sodium phosphate buffer (pH 6.2) containing 150 mmol/l NaCl. The column was washed until the ultraviolet (UV) absorbance curve returned to the baseline and stabilized. Baculovirus was eluted from the column in 1.5 mol/l NaCl containing sodium phosphate buffer (pH 8.0). The eluted fraction with the major peak was immediately diluted 10-fold with PBS to prevent inactivation of baculovirus particles. Eluted supernatants were concentrated by an ultracentrifuge at 80,000×g for 1.5 hours at 4ºC. Baculovirus pellets were dissolved in PBS (pH 7.0) containing 0.5% bovine serum albumin, and stored at −80°C.
Protein gel electrophoresis, silver staining, and immunoblotting
Baculovirus particles were lysed in Laemmeli sample buffer (Bio-Rad, Hercules, CA) containing protease inhibitor and beta-mercaptoethanol. Samples were heated at 95 ºC for 5 minutes to denature the proteins which were subjected to SDS–PAGE using a precast 12% Mini-PROTEAN TGX stain-free gel (Bio-Rad). Protein bands were visualized by staining the gel with a SilverXpress Silver Staining kit (Invitrogen).
For immunoblotting, gels were electroblotted onto a polyvinylidene fluoride (PVDF) membrane (Bio-Rad) in 25 mmol/l Tris, 192 mmol/l glycine, and 20% methanol.36,37 The membrane was incubated in Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) for one hour at room temperature and was probed with a baculovirus anti-gp64 monoclonal antibody (clone AcV5) (Santa Cruz Biotechnology, Dallas, TX) at 1:1,000 dilution for overnight incubation. The membrane was washed twice for 10 minutes in Tris–buffer saline (TBS), followed by incubation with a secondary antibody, goat antimouse infrared dye 800CW (LI-COR) at a 1:20,000 dilution for 1 hour. After thorough washing with TBS-Tween 20 (1%) and TBS, the immunoblot was visualized with an Odyssey Classic Imager equipped with Image Studio Software (LI-COR).
Transmission electron microscopy
For negative staining, a 10-μl drop of concentrated baculovirus sample was placed on a Formvar carbon-coated grid. After 30 seconds, most of the sample was removed using filter paper. The grid was rinsed with three drops of distilled water and placed in a 2% phosphotungstate acid solution for 30 seconds. The sample was allowed to dry and analyzed using a Transmission Electron Microscopy (TEM, Hitachi).
Statistical analysis
Statistical analysis was done using a two-tailed Student’s t-test. A P-value of ≤ 0.05 was considered statistically significant.
Acknowledgments
This work was supported in part by the start-up funding from Cincinnati Children’s Research Foundation (CCRF) to M.N. and Innovative Core Grant (ICG) to the CCRF Vector Production Facility.
The authors declare no conflict of interest.
The last two authors contributed equally to this work.
References
- Ikonomou, L, Schneider, YJ and Agathos, SN (2003). Insect cell culture for industrial production of recombinant proteins. Appl Microbiol Biotechnol 62: 1–20. [DOI] [PubMed] [Google Scholar]
- Contreras-Gómez, A, Sánchez-Mirón, A, García-Camacho, F, Molina-Grima, E and Chisti, Y (2014). Protein production using the baculovirus-insect cell expression system. Biotechnol Prog 30: 1–18. [DOI] [PubMed] [Google Scholar]
- Cox, MM (2012). Recombinant protein vaccines produced in insect cells. Vaccine 30: 1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce, FM and Bucher, NL (1996). Baculovirus-mediated gene transfer into mammalian cells. Proc Natl Acad Sci USA 93: 2348–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost, TA, Condreay, JP and Jarvis, DL (2005). Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol 23: 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimpel, AM and Buchanan, LK (1967). Human feeding tests using a nuclear-polyhedrosis virus of Heliothis zea. J Invertebr Pathol 9: 55–57. [DOI] [PubMed] [Google Scholar]
- Hu, YC (2008). Baculoviral vectors for gene delivery: a review. Curr Gene Ther 8: 54–65. [DOI] [PubMed] [Google Scholar]
- Airenne, KJ, Hu, YC, Kost, TA, Smith, RH, Kotin, RM, Ono, C et al. (2013). Baculovirus: an insect-derived vector for diverse gene transfer applications. Mol Ther 21: 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenoutis, C, Efrose, RC, Swevers, L, Lavdas, AA, Gaitanou, M, Matsas, R et al. (2006). Baculovirus-mediated gene delivery into Mammalian cells does not alter their transcriptional and differentiating potential but is accompanied by early viral gene expression. J Virol 80: 4135–4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkonen, KE, Airenne, K and Ylä-Herttulala, S (2015). Baculovirus-mediated gene delivery and RNAi applications. Viruses 7: 2099–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blissard, GW and Rohrmann, GF (1990). Baculovirus diversity and molecular biology. Annu Rev Entomol 35: 127–155. [DOI] [PubMed] [Google Scholar]
- Zeng, J, Du, J, Zhao, Y, Palanisamy, N and Wang, S (2007). Baculoviral vector-mediated transient and stable transgene expression in human embryonic stem cells. Stem Cells 25: 1055–1061. [DOI] [PubMed] [Google Scholar]
- Zeng, J, Du, J, Lin, J, Bak, XY, Wu, C and Wang, S (2009). High-efficiency transient transduction of human embryonic stem cell-derived neurons with baculoviral vectors. Mol Ther 17: 1585–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, LY, Chen, CL, Lin, SY, Li, KC, Yeh, CL, Chen, GY et al. (2014). Efficient gene delivery into cell lines and stem cells using baculovirus. Nat Protoc 9: 1882–1899. [DOI] [PubMed] [Google Scholar]
- Chen, CY, Lin, CY, Chen, GY and Hu, YC (2011). Baculovirus as a gene delivery vector: recent understandings of molecular alterations in transduced cells and latest applications. Biotechnol Adv 29: 618–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanbridge, LJ, Dussupt, V and Maitland, NJ (2003). Baculoviruses as vectors for gene therapy against human prostate cancer. J Biomed Biotechnol 2003: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, YK, Kwon, JT, Choi, JY, Jiang, HL, Arote, R, Jere, D et al. (2010). Suppression of tumor growth in xenograft model mice by programmed cell death 4 gene delivery using folate-PEG-baculovirus. Cancer Gene Ther 17: 751–760. [DOI] [PubMed] [Google Scholar]
- Cecchini, S, Virag, T and Kotin, RM (2011). Reproducible high yields of recombinant adeno-associated virus produced using invertebrate cells in 0.02- to 200-liter cultures. Hum Gene Ther 22: 1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allay, JA, Sleep, S, Long, S, Tillman, DM, Clark, R, Carney, G et al. (2011). Good manufacturing practice production of self-complementary serotype 8 adeno-associated viral vector for a hemophilia B clinical trial. Hum Gene Ther 22: 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten, OW, Charrier, S, Laroudie, N, Fauchille, S, Dugué, C, Jenny, C et al. (2011). Large-scale manufacture and characterization of a lentiviral vector produced for clinical ex vivo gene therapy application. Hum Gene Ther 22: 343–356. [DOI] [PubMed] [Google Scholar]
- Makkonen, KE, Turkki, P, Laakkonen, JP, Ylä-Herttuala, S, Marjomäki, V and Airenne, KJ (2013). 6-o- and N-sulfated syndecan-1 promotes baculovirus binding and entry into Mammalian cells. J Virol 87: 11148–11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasimuzzaman, M (2014). Heparan sulfate in baculovirus binding and entry of mammalian cells. J Virol 88: 4607–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasimuzzaman, M and Persons, DA (2012). Cell membrane-associated heparan sulfate is a receptor for prototype foamy virus in human, monkey, and rodent cells. Mol Ther 20: 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasimuzzaman, M, Lynn, D, Ernst, R, Beuerlein, R, Smith, RH, Shrestha, A et al. (2015). Production and purification of high-titer foamy virus vector for the treatment of leukocyte adhesion deficiency. Mol Ther Methods Clin Dev 1: 14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urabe, M, Nakakura, T, Xin, KQ, Obara, Y, Mizukami, H, Kume, A et al. (2006). Scalable generation of high-titer recombinant adeno-associated virus type 5 in insect cells. J Virol 80: 1874–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C and Wang, S (2012). A pH-sensitive heparin-binding sequence from Baculovirus gp64 protein is important for binding to mammalian cells but not to Sf9 insect cells. J Virol 86: 484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, C, Soh, KY and Wang, S (2007). Ion-exchange membrane chromatography method for rapid and efficient purification of recombinant baculovirus and baculovirus gp64 protein. Hum Gene Ther 18: 665–672. [DOI] [PubMed] [Google Scholar]
- Bess, JW Jr, Gorelick, RJ, Bosche, WJ, Henderson, LE and Arthur, LO (1997). Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virol 230: 134–144. [DOI] [PubMed] [Google Scholar]
- Michalsky, R, Passarelli, AL, Pfromm, PH, and Czermak, P (2010).Concentration of the baculovirus autographa californica M nucleopolyhedrovirus (AcMNPV) by ultrafiltration. Desalination 250: 1125–1127. [Google Scholar]
- Vicente, T, Peixoto, C, Carrondo, MJ and Alves, PM (2009). Purification of recombinant baculoviruses for gene therapy using membrane processes. Gene Ther 16: 766–775. [DOI] [PubMed] [Google Scholar]
- Eaves, GN and Jeffries, CD (1963). Isolation and properties of an exocellular nuclease of serratia marcescens. J Bacteriol 85: 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorio, H, Tran, R, Meghrous, J, Bourget, L and Kamen, A (2006). Analysis of baculovirus aggregates using flow cytometry. J Virol Methods 134: 8–14. [DOI] [PubMed] [Google Scholar]
- Floyd, R and Sharp, DG (1979). Viral aggregation: buffer effects in the aggregation of poliovirus and reovirus at low and high pH. Appl Environ Microbiol 38: 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney, D, McCoy, M, Gordon, N and Afeyan, N (1998). Characterization of large-pore polymeric supports for use in perfusion biochromatography. J Chromatogr A 807: 165–184. [DOI] [PubMed] [Google Scholar]
- Chalabi, N, Maurizis, JC, Le Corre, L, Delort, L, Bignon, YJ and Bernard-Gallon, DJ (2005). Quantification by affinity perfusion chromatography of phosphorylated BRCAl and BRCA2 proteins from tumor cells after lycopene treatment. J Chromatogr B Analyt Technol Biomed Life Sci 821: 188–193. [DOI] [PubMed] [Google Scholar]
- Nasimuzzaman, M, Waris, G, Mikolon, D, Stupack, DG and Siddiqui, A (2007). Hepatitis C virus stabilizes hypoxia-inducible factor 1alpha and stimulates the synthesis of vascular endothelial growth factor. J Virol 81: 10249–10257. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nasimuzzaman, M, Kuroda, M, Dohno, S, Yamamoto, T, Iwatsuki, K, Matsuzaki, S et al. (2005). Eradication of Epstein-Barr virus episome and associated inhibition of infected tumor cell growth by adenovirus vector-mediated transduction of dominant-negative EBNA1. Mol Ther 11: 578–590. [DOI] [PubMed] [Google Scholar]