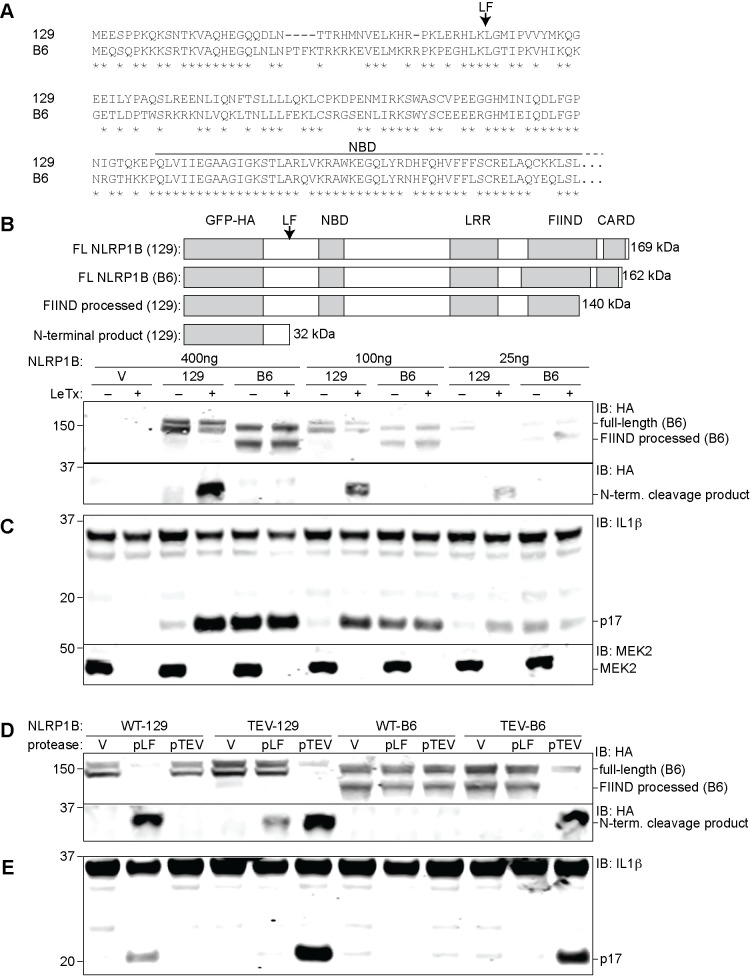
Fig 1. The B6 variant of NLRP1B is not cleaved by LF but can form an inflammasome in response to proteolysis.
(A) The amino acid sequence of the first 244 residues of NLRP1BB6 was aligned to the homologous sequence of NLRP1B129. The arrow above the alignment indicates the LF-cleavage site in NLRP1B129. Asterisks indicate sites of amino acid identity. (B) For detection of NLRP1B expression, 293T cells were transfected with the indicated amounts of and empty vector (V) or plasmids encoding GFP-HA-NLRP1B129 or GFP-HA-NLRP1BB6 (construct schematics and the predicted molecular weight of each protein is depicted in the upper panel). Cells were treated overnight with anthrax lethal toxin protein (LeTx, 1μg/ml) 24h post-transfection, and then lysates were analyzed by immunoblotting (IB) with the indicated antibodies. For NLRP1 expression and cleavage, CASP1 and IL1B were omitted to prevent cell death and resulting apparent differences of expression. For blots probed with anti-HA (NLRP1B), the lysates were not boiled prior to loading to prevent aggregation and smearing of full-length and FIIND-processed NLRP1B on the immunoblot. To visualize the N-terminal processed form of NLRP1B, the lysates were boiled and resolved on a separate gel. (C) 293T Cells were transfected with the same amount of titrated NLRP1B encoding plasmid and treated as in B, but transfections also included plasmids encoding mouse CASP1 (200ng), IL-1β (200ng) and 200ng of empty vector. (D) For detection of NLRP1B expression, 293T cells were transfected for 36h with 250ng of plasmids encoding WT 129 or B6 NLRP1B or mutants engineered to express the TEV-protease site. Each plasmid was co-transfected with either 100ng of empty vector (V) or plasmids encoding TEV-protease (pTEV) or lethal factor protease (pLF), supplemented with 300ng empty vector (pMSCV). Cells were not treated with LeTx and lysates were analyzed by immunoblotting as in (B). (E) Cells were treated as in C, but with 8ng of plasmids encoding NLRP1B, along with 200ng of plasmids encoding mCASP-1 and mIL-1β, and 200ng empty vector to normalize plasmid quantities. Lower quantities of NLRP1B were transfected as compared to panel D so to avoid spontaneous NLPR1B activation. For panels B-E, data shown are representative of at least three similar experiments.