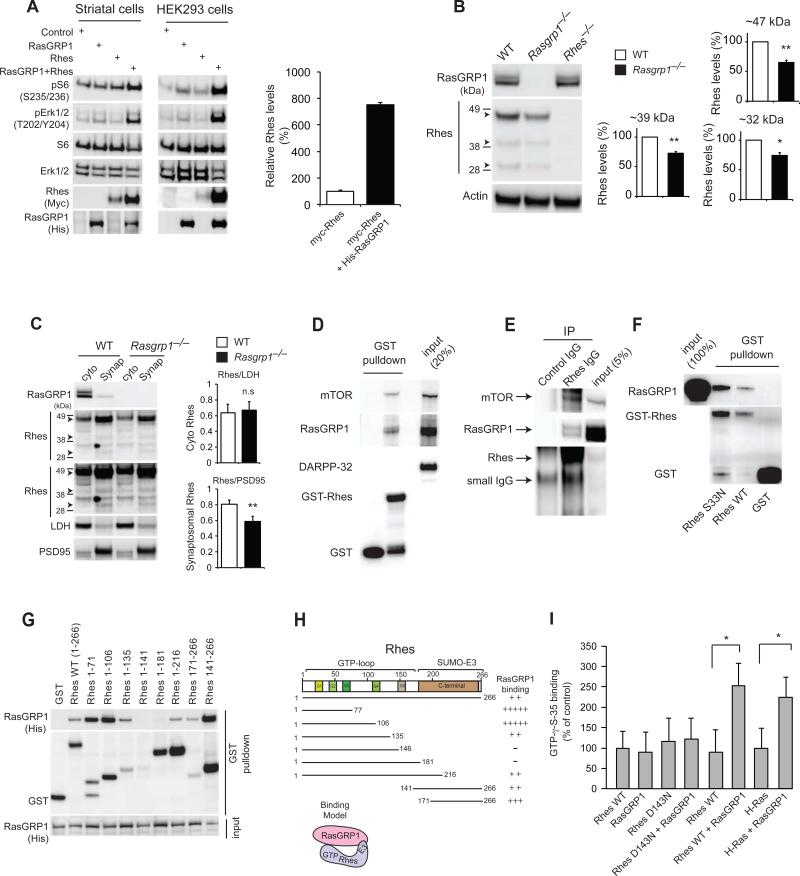
Figure 1. RasGRP1 interacts with Rhes in the striatum.
(A) Western blot analysis of the abundance of myc-tagged Rhes, phosphorylated and total S6, ERK, and other indicated proteins in cultured striatal neuronal cells (STHdhQ7/Q7) and HEK293 cells transfected with His-tagged RasGRP1. Right, quantification of Rhes in striatal cells. (B). Western blot analysis of Rhes (47kD, 39kD, and 32kD, arrowheads) in striatal lysates from wild-type and Rasgrp1−/− mice. (C). Cytoplasmic (cyto) and synaptosomal (synap) separation of Rhes from the striatum of wild-type and Rasgrp1−/− mice. Cytosolic (LDH) and synaptosomal (PSD-95) markers are indicated. (D) Western blot analysis of GST-Rhes interaction with endogenous RasGRP1, mTOR or DARPP-32 in the striatal lysates. (E) Immunoprecipitation (IP) of Rhes with Rhes IgG or control IgG and Western blotting for endogenous mTOR or RasGRP1. (F) Western blotting to assess the interaction of purified RasGRP1 with wild-type or S33N mutant GST-Rhes in vitro. (G) Domain interaction of GST-Rhes wild-type or various Rhes fragments with overexpressed His-RasGRP1 in HEK293 cells. (H) Interaction model showing two-point interaction (N-terminal and C-terminal E3 ligase domain) of Rhes with RasGRP1. (I) Radiolabelling filter-binding GEF assay was performed using recombinant RasGRP1 and Rhes (wild-type, D143N) or H-Ras proteins. The amount of GTPγS-35 bound on membrane was imaged and signals were quantified. Data are means ± SEM, n=3-5 experiments. *p<0.05 and **p<0.01 by a Student's t-test.