Figure 2.
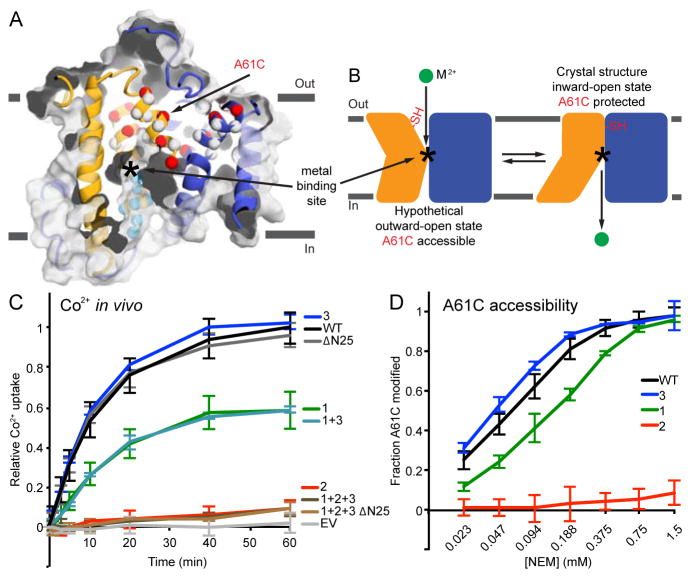
Cysteine accessibility scanning reveals the outward metal permeation pathway that is sealed shut in our crystallization construct. (A) Internal slice of the inward-facing DraNramp structure, including solvent accessibility of a panel of cysteine mutants spanning TM1, 3, and 6 using NEM. Spheres show Cα positions of highly NEM-protected (gray), outward-accessible (also MTSET and MTSEA-modified; red), inward-accessible (also MTSEA- but not MTSET- modified; cyan), or only NEM-accessible (black) residues. Accessibility is assessed as >50% NEM-modification in at least two separate experiments. Many outward-accessible residues, including A61C, are buried in our inward-open structure, suggesting they line an aqueous passage to the metal-binding site (approximate location labeled *) in an alternate outward-open conformation. (B) DraNramp’s proposed conformational equilibrium, in which A61C is solvent-accessible in the outward-open state, but buried (and thus NEM-protected) in the inward-open conformation. (C) The patch mutants in the crystallized DraNramp construct, tested alone or in combinations, have varying effects on in vivo Co2+ transport. While the 25-residue N-terminal truncation and patch 3 (RR398-9HH) did not impair function, patch 1 (QK169-70HH) reduced transport and patch 2 (EEK251-3YYY) completely eliminated transport. (D) While the transport-competent patch 1 and patch 3 mutants retained A61C accessibility (patch 1 at a reduced level), the transport-dead patch 2 mutant eliminated A61C accessibility, suggesting it locks the protein in the inward-open state. All data are averages ± s.d. (n ≥ 3). For reference, WT and EV Co2+ uptake time course and WT A61C accessibility data are repeated in subsequent figures. See also Figure S2 and Table S1.