Figure 4.
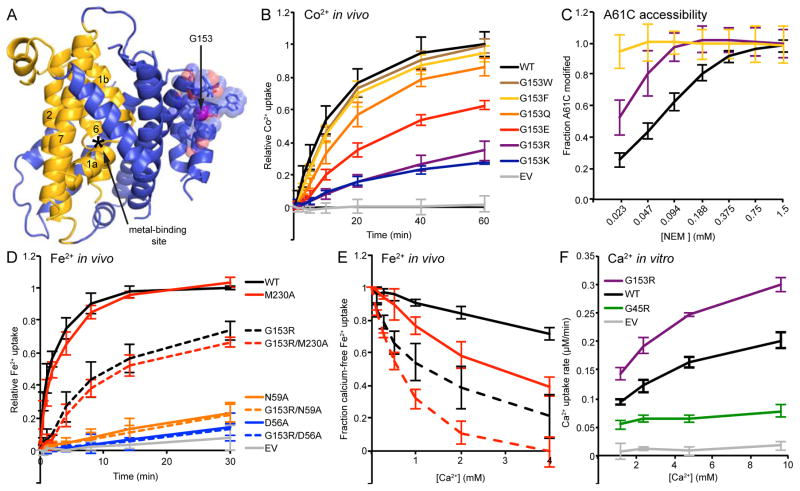
G153R mutation perturbs outward-facing state and alters metal selectivity of binding site to favor Ca2+. (A) In the inward-facing DraNramp structure, G153 is located on the extracellular end of TM4 tightly packed in the back of the scaffold, far from the conserved metal-binding site. The G153 Cα is a magenta sphere; nearby residues are shown as transparent spheres with sidechains as sticks. (B) Co2+ uptake data showing charged bulky substitutions at G153 reduced transport, while aromatic substitutions retained WT-level transport. (C) G153R showed increased accessibility to NEM modification of the A61C outward-open conformational reporter, and G153F showed a further increase. (D) In in vivo Fe2+ uptake, G153R showed little additional effect when combined with metal-binding site mutations, and its residual Fe2+ transport still uses the conserved metal-binding site as double mutations G153R/D56A and G153R/N59A both severely reduced transport. (E) Competing Ca2+ reduced in vivo Fe2+ uptake more for G153R than WT, and double mutant G153R/M230A was even more susceptible to Ca2+ competition. The Ca2+-free Fe2+ uptake level (2 min uptake for WT/M230A; 15 min uptake for G153 mutants) was set to 100% for each variant to facilitate direct % inhibition comparison. (F) G153R transported more Ca2+ (as detected by Fura-2) than WT or the G45R mutant in an in vitro proteoliposome assay. All data are averages ± s.d. (n ≥ 3). See also Figure S4.