Figure 5.
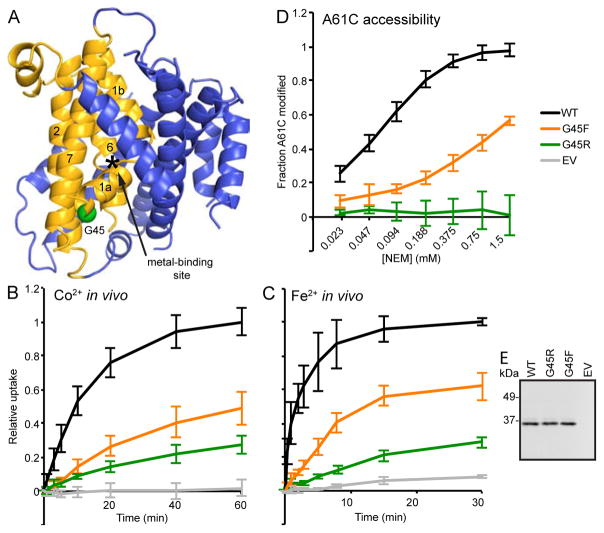
G45R mutation inhibits transport by locking Nramp in outward-closed conformation. (A) G45 (green sphere) is on TM1a on the intracellular side of the bundle, 11 residues below the metal-binding D56. (B) G45R and G45F were significant impaired in in vivo Co2+ transport. (C) G45F also impaired in vivo Fe2+ transport (WT, G45R and EV data reproduced from Figure 3E for comparison). (D) G45R and G45F drastically decreased accessibility of outside-open conformational reporter A61C, indicating a strong preference for the inward-open state. (E) Western blot showing G45R and G45F expressed similarly to WT DraNramp. All data are averages ± s.d. (n ≥ 3).