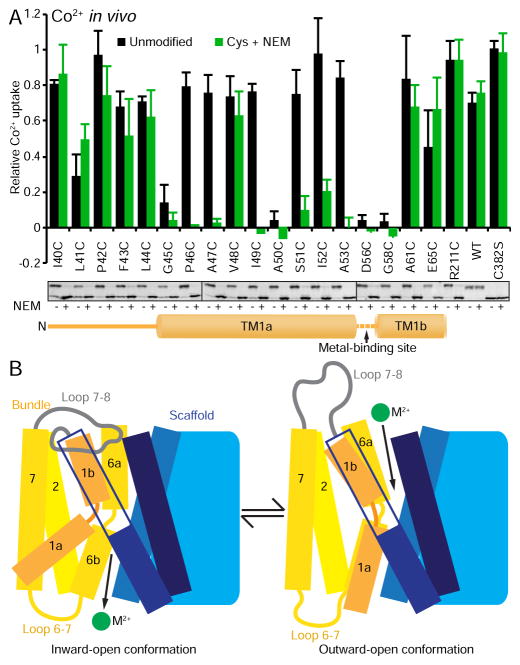
Figure 7.
TM1a movement is essential to the conformational change that opens the outward metal permeation pathway, thus enabling Nramp metal transport. (A) Initial (6 min) in vivo Co2+ transport (minus EV control) for accessible single-cysteine mutants along TM1 that were either left unmodified (black bars) or pre-reacted with NEM (3 mM; green bars). NEM modification greatly impaired transport for six out of the seven TM1a positions where cysteine mutants had high transport activity. Data are averages ± s.d. (n = 3). Western blots show all introduced cysteines were efficiently NEM-labeled, as preincubation with NEM (+) prevented formation of PEG5K-maleimide DraNramp [upper band in the (−) lanes]. R211C on extracellular loop 5-6 was readily NEM-modified without affecting activity. Endogenous C382 on TM10 in WT was not modified by NEM, and thus was fully modified by PEG5K-maleimide. The cysteine-less C382S is not labeled by either NEM or 5KPEG-maleimide. (B) Model of the conformational change process in DraNramp. Our metal transport and cysteine accessibility results demonstrated that the unencumbered TM1a movement is essential for conformational change into the outward-facing state, including evicting loop 7-8 which caps the outside metal permeation pathway and opening the interface between the bundle (TM1b and 6a) and scaffold (TM3, 8, and 10), thus allowing periplasmic metal ions to reach the binding site in the unwound regions of TM1 and 6 at the center of the membrane plane. See also Figure S6.