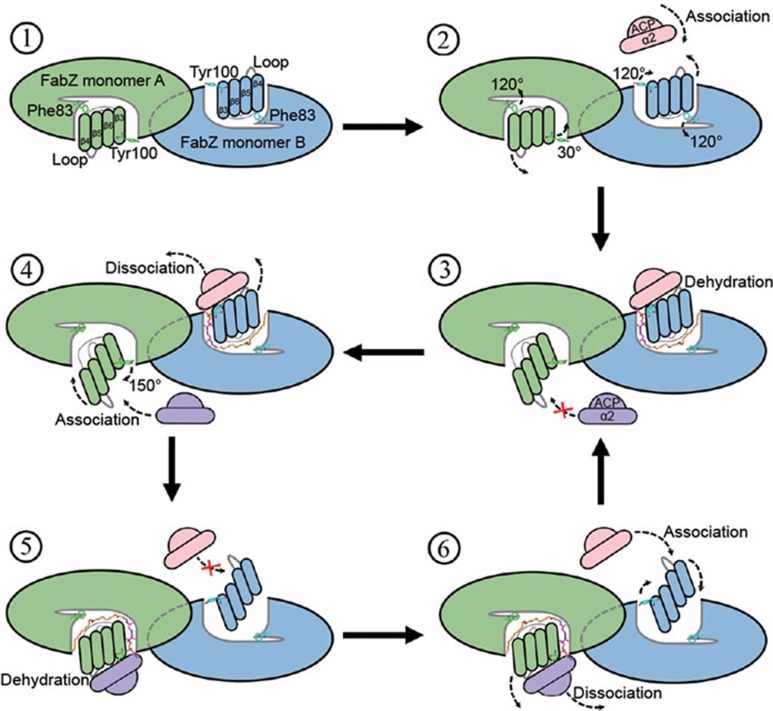
Figure 6.
Schematic diagram of the dynamic seesaw-like continuous catalytic mechanism model of FabZ during fatty acid biosynthesis. Monomers A and B within a FabZ dimer subunit and two ACP molecules are colored in green, light blue, wheat and purple, respectively. Four critical β-strands from the β-sheet layer are shown as oval rafts. Key gatekeeper residues Tyr100 and Phe83 from FabZ monomer B (cyan) and monomer A (green) are shown as sticks. The 4′-phosphopantetheine arm (magenta) attached with a long fatty acid chain (orange) from ACP are shown as lines. In the native conformation of FabZ, the sidechains of Tyr100 and Phe83 adopt close conformation to protect the hydrophobic catalytic tunnel from bulk solvent (step 1). When the first ACP molecule (wheat) binds to FabZ monomer B, the L-shaped active tunnel is expanded to a Y-shape due to movement of the β-sheet layer toward 4. The sidechains of Tyr100 and Phe83 flop ∼120° to an open conformation, which allows the substrate chain carried by the 4′-Pan-arm to insert into the active tunnel for catalytic dehydration. Meanwhile, the β-sheet layer in the ACP-unbound FabZ monomer A moves further toward β4 due to a ∼30° rotation of the Tyr100 sidechain from its normal closed conformation to form an abnormal conformation unfavorable for ACP binding (purple) (steps 2 and 3). When the dehydration reaction is finished on monomer B, the β-sheet layer moves further toward β4 to release ACP, whereas the β-sheet layer on monomer A moves back with a ∼150° rotation of the Tyr100 sidechain to a normal open conformation to accept binding and catalysis of the second ACP (steps 4 and 5). By doing so, the catalysis core is exchanged from FabZ monomer B to monomer A. When the second dehydration reaction is finished, the catalysis core is exchanged to B again like a see-saw due to the movements of the β-sheet layer (steps 4-6), which causes the high-efficiency catalytic dehydration mechanism of FabZ in the FAS system.