Abstract
Cells and organisms adapt to mitochondrial dysfunction by activating the mitochondrial unfolded protein response (UPRmt), which is regulated by mitochondrial-to-nuclear communication; and UPRmt activation can also be transmitted between different cell types suggesting a role in tissue coordination. Shao and colleagues now identify a neuronal circuit and a secreted neuropeptide required for cell non-autonomous UPRmt regulation.
Mitochondrial dysfunction can originate from genetic lesions or pathogen-produced toxins that impair mitochondrial function (oxidative phosphorylation, etc.), as well as by proteotoxic effects within the organelle. One response employed to alleviate mitochondrial stress is the UPRmt, a transcriptional response of over 500 transcripts that promotes the repair and recovery of defective mitochondria, metabolic adaptations, xenobiotic detoxification and innate immunity. In C. elegans, the UPRmt is regulated by the transcription factor ATFS-11 coupled with extensive chromatin remodeling2. ATFS-1 activity is regulated by organelle partitioning and mitochondrial protein import efficiency. ATFS-1 is expressed in all tissues and imported into healthy mitochondria where it is degraded. However, if mitochondrial function is perturbed, protein import efficiency is reduced, causing ATFS-1 to accumulate in the cytosol. Because ATFS-1 also has a nuclear localization sequence, it then traffics to the nucleus to activate the transcriptional response.
In addition to intracellular signaling, UPRmt activation within neurons can be communicated to distal tissues such as the intestine to activate the response in these cells. Non-autonomous UPRmt activation likely promotes coordination of mitochondrial function or metabolic adaptations across tissues, which may contribute to the metabolic abnormalities found in distal tissues in neurodegenerative diseases such as Huntington's disease and Parkinson's disease3. Because neurons do not innervate the worm intestine, non-automonous UPRmt signaling has been hypothesized to be mediated by a “mitokine”4, however, the mode of signal transduction has remained unclear, as have the functional ramifications.
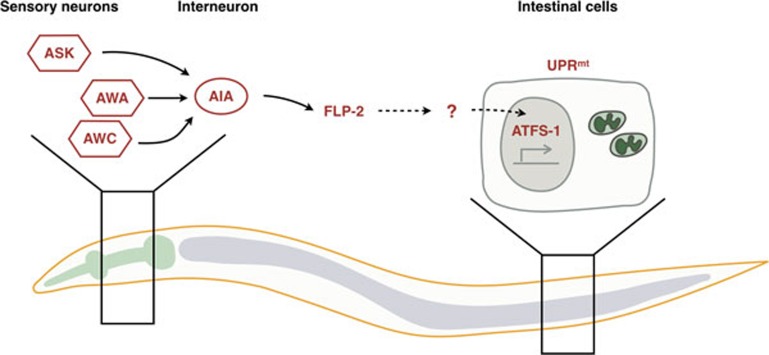
Using an impressive variety of cell type-specific CRISPR techniques, Shao and colleagues now demonstrate that multiple forms mitochondrial dysfunction (impairment of a mitochondrial protease, the respiratory chain, localized reactive oxygen species or depletion of inner membrane potential) administered specifically within neurons are capable of stimulating UPRmt activation in neurons5. Interestingly, all of the neuronal mitochondrial stresses other than membrane potential depletion caused subsequent intestinal cell UPRmt activation suggesting that not all forms of mitochondrial dysfunction activate the non-autonomous UPRmt. The robust system was used to identify a sub-neuronal circuit required for propagation of the UPRmt between tissues. The neural circuit required three sensory neurons capable of sampling the environment and the AIA interneuron, which receives and integrates information from sensory neurons for downstream signaling (Figure 1). Because the interneuron does not physically interact with the intestine, the authors considered secreted signaling molecules such as neuropeptides. The requirement for a neuropeptide processing protease for non-autonomous UPRmt induction strongly supported the involvement of at least one of the ∼250 known neuropeptide(s) in C. elegans.
Figure 1.
Cell non-autonomous regulation of the UPRmt. Mitochondrial perturbations within three C. elegans sensory neurons (ASK, AWA, AWC) causes activation of ATFS-1 and the UPRmt within these cell types and also within intestinal cells. Transmission of a signal between the sensory neurons requires an interneuron (AIA), the secreted neuropeptide FLP-2 as well as ATFS-1 within the intestinal cells. It is currently unclear how secreted FLP-2 affects intestinal ATFS-1.
An elegant neuron-specific CRISPR screen identified six neuropeptides required for UPRmt propagation across tissues, but only one of these was sufficient. Similar to neuron-specific activation of ATFS-1, neuron-specific expression of FLP-2 was sufficient to induce the UPRmt in the intestine. Further, deletion of flp-2 within a single interneuron impaired non-autonomous UPRmt activation initiated within the sensory neurons. While the UPRmt-mitokine story is not complete, the discovery of a sub-neuronal circuit and a secreted neuropeptide by Shao and colleagues represents a significant step forward allowing the field to focus on events downstream of the interneuron and FLP-2 secretion.
FLP-2 may well be a peptide secreted during mitochondrial stress to affect other cell types, which fits the definition of a mitokine. However, those cell types and the receptor remain to be identified as expression of FLP-2 specifically by intestinal cells was not sufficient to activate the intestinal UPRmt5. Thus, FLP-2 likely functions elsewhere. Similarly, a recent study demonstrated a requirement for the neuronal release of serotonin in activation of the non-autonomous UPRmt, but how serotonin effects intestinal cells remains to be determined3. While the relationship between FLP-2 and serotonin has not been examined, it is safe to assume that a number of interesting signaling events occur downstream of both molecules to affect the regulation of intestinal ATFS-1. Both studies indicate that ATFS-1 is essential in both the sensing neurons as well as in the intestinal cells for non-autonomous UPRmt activation3,5. In neurons where mitochondria are perturbed directly, ATFS-1 activation is likely achieved by impaired mitochondrial protein import efficiency. But, how is ATFS-1 activated in the receiving intestinal cells? Does non-autonomous signaling perturb mitochondrial function within the intestine or somehow engage ATFS-1 independent of mitochondrial dysfunction? Alternatively, non- autonomous signaling may promote chromatin rearrangements in intestinal cells, which has been shown to activate ATFS-1 and the UPRmt2.
It will also be important to determine the functional effects of non-autonomous UPRmt signaling. Stated differently, in what physiologic scenarios do sensory neurons first encounter mitochondrial stress and what benefit is conferred by UPRmt activation in distal tissues? The authors suggest a role in sensing mitochondrial stress, which may propagate a signal to distal tissues to “pre”-activate or prime a response. Given the role of the UPRmt in innate immunity and responding to pathogens that produce mitochondrial toxins, this may provide a means to activate the UPRmt in intestinal cells before the pathogen has accumulated to a level within the intestinal lumen that severely perturbs mitochondrial function6,7. Alternatively, because UPRmt activation shifts metabolism from oxidative phosphorylation to glycolysis to facilitate mitochondrial repair7, non-autonomous signaling may simply coordinate metabolism between different tissues or organs. Consistent with this idea, the mammalian hormone Fgf21 has been suggested to be a mitokine as its secretion is induced during mitochondrial stress via the stress-activated transcription factor ATF4. In this context, Fgf21 produced by muscle cells promotes mitochondrial biogenesis and fat browning in adipocytes along with enhanced lipid catabolism8.
References
- Nargund AM, Pellegrino MW, Fiorese CJ, et al. Science 2012; 337:587–590. [DOI] [PMC free article] [PubMed]
- Tian Y, Garcia G, Bian Q, et al. Cell 2016; 165:1197–1208. [DOI] [PMC free article] [PubMed]
- Berendzen KM, Durieux J, Shao LW, et al. Cell 2016; 166:1553–1563. [DOI] [PMC free article] [PubMed]
- Durieux J, Wolff S, Dillin A. Cell 2011; 144:79–91. [DOI] [PMC free article] [PubMed]
- Shao LW, Niu R, Liu Y. Cell Res 2016; 26:1182–1196. [DOI] [PMC free article] [PubMed]
- Liu Y, Samuel BS, Breen PC, et al. Nature 2014; 508:406–410. [DOI] [PMC free article] [PubMed]
- Pellegrino MW, Nargund AM, Kirienko NV, et al. Nature 2014; 516:414–417. [DOI] [PMC free article] [PubMed]
- Lin YF, Haynes CM. Mol Cell 2016; 61:677–682. [DOI] [PMC free article] [PubMed]
- Kim KH, Jeong YT, Oh H, et al. Nat Med 2013; 19:83–92. [DOI] [PubMed]