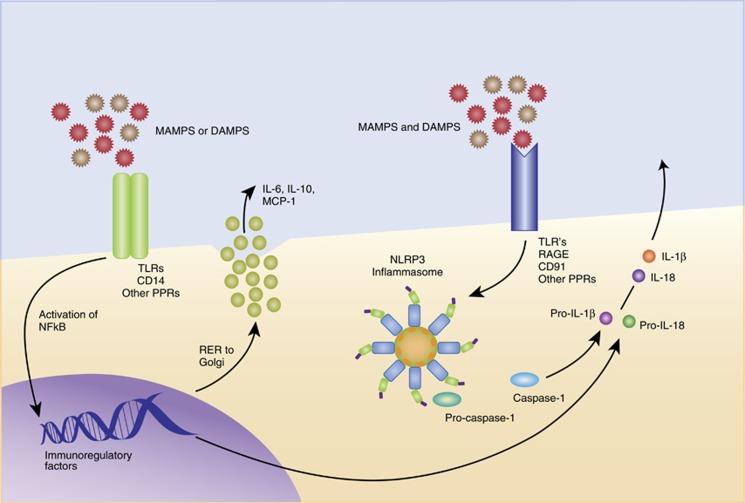
Figure 3.
Both single signal (inflammasome-independent) and double signal (inflammasome-dependent) sterile inflammation. Inflammasome-dependent cytokine synthesis and release is a two-step process that is initiated after ligation of Toll-like receptor (TLRs) and other receptors capable of binding danger-associated molecular patterns (DAMPs) and/or microbial-associated molecular patterns (MAMPs) leading to NLRP3 (nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain protein 3) gene transcription, translation, and protein production (Duewell et al, 2010). Once sufficient NLRP3 protein has been formed, then a second activation signal is needed that leads NLRP3 to interact with the adaptor protein ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), which then recruits procaspase-1 through its caspase recruitment domain. This assembly of proteins is considered the inflammasome, and once formed triggers the activation/cleavage of procaspase 1. Mature, active caspase-1 then acts to cleave pro-interleukin-1β (IL-1β) into mature IL-1β (and IL-18) protein. The NLRP3 inflammasome responds to a broad range of signals, for example, ATP, K+ efflux, β-amyloid, silica, uric acid crystals, and reactive oxygen species (ROS) (Elliott and Sutterwala, 2015). In addition, there are several candidate receptors capable of binding DAMPs and MAMPs, including TLRs, RAGE, and CD91 (Asea et al, 2002; Calderwood et al, 2007a, b). This figure was adapted from a figure courtesy of Tom Maslanik, PhD/MBA.