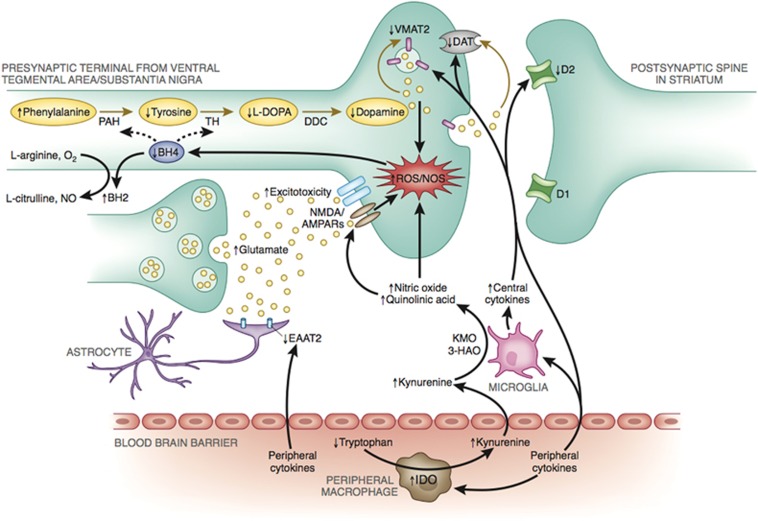
Figure 2.
Potential mechanisms of inflammation effects on dopamine (DA) synthesis, release, and receptor signaling. Evidence indicates that inflammation and release of cytokines from the periphery, or those produced locally by activated microglia or infiltrating macrophages, can produce nitric oxide, as well as quinolinic acid through indoleamine 2,3-dioxygenase (IDO) and kynurenine pathways, both of which contribute to oxidative stress, and reactive oxygen species (ROS) generation. Increased ROS and inflammation-induced nitric oxide contribute to oxidation of tetrahydrobiopterin (BH4), a cofactor required for the conversion of phenylalanine to tyrosine and tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA), which are necessary for the synthesis of DA. Furthermore, some evidence exists that inflammatory cytokines may decrease the expression or function of the vesicular monoamine transporter 2 (VMAT2) and/or increase expression or function of the dopamine transporter (DAT). Dysregulation of DAT and vesicular packaging mechanisms can increase cytosolic DA, leading to auto-oxidation and generation of ROS and neurotoxic quinones. In addition, inflammation-induced increased release and decreased reuptake of glutamate by glial cells, combined with quinolinic acid activation of N-Methyl-D-aspartic acid receptors, may lead to glutamate excitotoxicity that further contributes to oxidative stress and decreased DA availability. Finally, inflammatory cytokines may also decrease DA signaling by reducing DA D2 receptors. Figure adapted from Felger and Miller, 2012. 3-HAO, 3-hydroxyanthranilic acid oxygenase; AMPAR, 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl) propanoic acid receptor; D1, dopamine 1 receptor 1; D2, dopamine 2 receptor; DDC, dopamine decarboxylase; KMO, kynurenine 3-monooxygenase; NMDAR, N-methyl-D-aspartic acid receptor; NO, nitric oxide; NOS, nitric oxide synthase; PAH, phenylalanine hydroxylase; ROS, reactive oxygen species; TH, tyrosine hydroxylase.