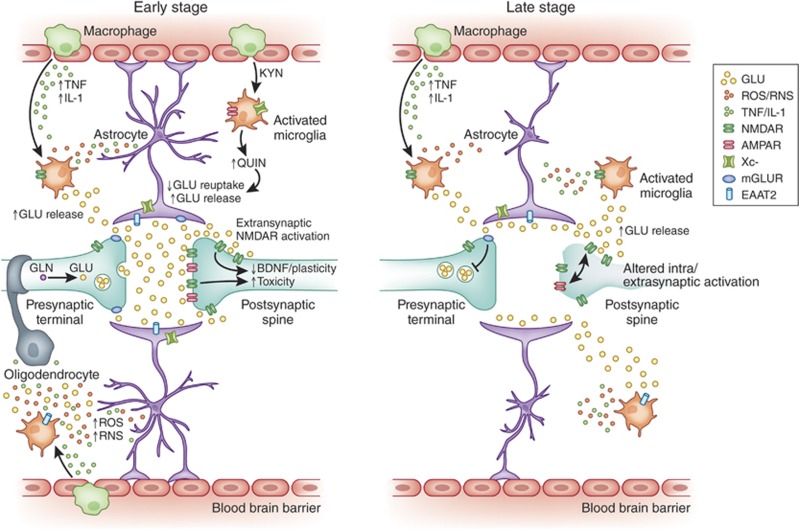
Figure 1.
Impact of inflammation on glutamate neurotransmission and synaptic integrity. Left panel depicts the glutamatergic synapse at an early stage of inflammation. High levels of inflammatory cytokines such as tumor necrosis factor (TNF) and interleukin (IL)-1β released by activated inflammatory cells including microglia, astroglial, and macrophages lead to increases in the concentration of synaptic glutamate (GLU), toxic kynurenine molecules with glutamate-like effects such as quinolinic acid (QUIN), and reactive oxygen (ROS)/nitrogen (RNS) species leading to oxidative stress. Effects of inflammatory molecules upon astrocytic cell morphology leads to decreased ability to ‘cradle', sequester, and contain glutamate within the synapse resulting in a spillover of the glutamate into the extrasynaptic space. Concurrent decreases in the number and functioning of excitatory amino-acid transporters (EAAT) 2-induced by inflammation further limits the ability of astrocytes to buffer and clear the spillover glutamate. Additional release of glutamate by increased xC-activity alone or in combination with reverse effluxive release via EAATs by immune-activated glial cells further adds to the concentrations of extrasynaptic glutamate available to diffuse and bind to extrasynaptic binding sites. Increases in synaptic glutamate resulting from early inflammatory changes are shown as leading to the overactivation of intrasynaptic ionotropic receptors such as α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate receptors (NMDA) potentially contributing to excitotoxicity. The spillover glutamate is also shown as binding to the extrasynaptic NMDA receptors leading to suppression of neurotrophic support from factors such as brain-derived neurotrophic factor (BDNF). Some of the excess glutamate is also seen binding to presynaptic metabotropic glutamate receptors (mGluR2/3), which provides negative feedback to block further glutamate release as shown in the right panel. Right panel depicts the glutamate synapse at a late stage of chronic inflammation. The progression of glutamatergic dysfunction has resulted in an overall loss of glutamate neurotransmission. Sustained stimulation of presynaptic mGluR2/3 systems by glutamate diffusing through extrasynaptic space leads to inhibition of excitatory neurotransmission and increased trapping of glutamate in the vesicles within the presynaptic membrane. The toxic effect of overstimulation of intrasynaptic AMPA during early immune activation has led to a downregulation, desensitization, and atrophic loss of intrasynaptic AMPA receptors. Decreasing intrasynaptic glutamatergic activity also leads to the loss of intrasynaptic NMDA signaling even while the extrasynaptic NMDA signaling continues to remain high. This altered ratio of intrasynaptic to extrasynaptic signaling leads to atrophy and regional loss of neurons seen in patients with mood disorders as seen in the shrinking postsynaptic neuron. Finally, the persistently increased extrasynaptic glutamate, increased ROS/RNS, and QUIN also contribute to astrocytic and oligodendrocyte toxicity leading to atrophic changes in these cells as well.