Abstract
The evidence to date, coupled with advances in immunology and genetics has afforded the field an unparalleled opportunity to investigate the hypothesis that a subset of patients with schizophrenia may manifest an immunophenotype, toward new potential diagnostics and therapeutics to reduce risk, alleviate symptoms, and improve quality of life in both at-risk populations and patients with established schizophrenia. In this paper, we will first summarize the findings on immune dysfunction in schizophrenia, including (1) genetic, prenatal, and premorbid immune risk factors and (2) immune markers across the clinical course of the disorder, including cytokines; C-reactive protein; immune cells; antibodies, autoantibodies and comorbid autoimmune disorders; complement; oxidative stress; imaging of neuroinflammation; infections; and clinical trials of anti-inflammatory agents and immunotherapy. We will then discuss a potential mechanistic framework toward increased understanding of a potential schizophrenia immunophenotype. We will then critically appraise the existing literature, and discuss suggestions for the future research agenda in this area that are needed to rigorously evaluate this hypothesis.
Introduction
The investigation of immune system abnormalities in schizophrenia, though ongoing for decades, has more recently become a popular area of research. This interest has been at least partially stimulated by our increased understanding of interactions between the immune system and the brain in other chronic medical disorders. Advances in genetics have led to the identification of associations between genes involved in the regulation of the immune system and increased risk of schizophrenia (Psychiatric Genomics Consortium, 2014; Shi et al, 2009). Prenatal maternal infection with a variety of different infectious agents is a replicated risk factor for schizophrenia in the offspring (Brown and Derkits, 2010), and may act synergistically with genetic factors on schizophrenia risk (Clarke et al, 2009). Autoimmune disorders and severe infections are associated with schizophrenia risk, and these associations appear to be bidirectional (Benros et al, 2014; Nielsen et al, 2014). There is evidence that patients with schizophrenia have immunologic abnormalities in the blood, cerebrospinal fluid (CSF), and central nervous system (CNS), including immune cell numbers, inflammatory markers, oxidative stress, and antibody titers (Kirkpatrick and Miller, 2013). Patients with schizophrenia may have an increased the prevalence of certain comorbid infections (Laney et al, 2015; Miller et al, 2013). Several trials have found that treatment with non-steroidal anti-inflammatory drugs (NSAIDs) or agents with anti-inflammatory properties, in adjunct to antipsychotics, may be associated with significant improvement in psychopathology in some patients with schizophrenia (Nitta et al., 2013; Sommer et al, 2014), and baseline blood levels of inflammatory markers may predict response to these agents (Laan et al, 2010; Muller et al, 2004). Taken together, findings suggest we need to more extensively and systematically evaluate the hypothesis that immune dysfunction may be involved in the pathogenesis of schizophrenia disorders.
A recent, seminal finding in neuroscience has promulgated our energy and enthusiasm regarding the immune hypothesis of schizophrenia. Sekar et al (2016) linked together how a specific variant of the C4 gene, which encodes the complement protein C4, may increase schizophrenia risk by influencing synaptic pruning during critical periods of the brain development (Sekar et al, 2016). This work connects replicated, yet seemingly disparate, findings that schizophrenia is associated with immune genes, has a usual age of onset in young adulthood, and is associated with cortical thinning and synapse loss.
It should be emphasized, however, that there is significant heterogeneity in the literature regarding findings for immune dysfunction in schizophrenia. For example, although some findings of altered peripheral blood immune markers have been frequently replicated, there are also numerous negative studies. There are many potential explanations for these discrepancies related to small sample sizes, the stage of the illness, medication status, and comorbid conditions. Another important potential explanation for these heterogeneous findings is that immune system dysfunction occurs in only a subset of patients with schizophrenia. This may reflect an inherent limitation of our phenomenologically based nosology. Recently, the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) identified three more homogeneous, neurobiologically distinct phenotypes, termed biotypes, of patients with psychosis that did not respect DSM clinical symptom-based diagnostic categories (Clementz et al, 2015). Just as there are many different underlying etiologies of fever, the B-SNIP study illustrates how distinct etiological pathways may lead to clinically similar symptomatology, namely, psychosis.
The evidence to date, coupled with advances in immunology and genetics, has afforded the field an unparalleled opportunity to investigate the hypothesis that a subset of patients with schizophrenia may manifest an immunophenotype, toward new potential diagnostics and therapeutics to reduce risk, alleviate symptoms, and improve quality of life in both at-risk populations and patients with established schizophrenia. In this paper, we will first summarize the findings on immune dysfunction in schizophrenia. We then discuss a potential mechanistic framework toward increased understanding of a potential immunophenotype of schizophrenia. We will then critically appraise the existing literature, and discuss suggestions for the future research agenda in this area that are needed to rigorously evaluate this hypothesis, including more careful definition of sample populations, the use of longitudinal studies, identification of inflammation in the brain, and the response to specific targeting of the immune system.
Genetic, prenatal, and premorbid immune risk factors for schizophrenia
Immunogenetic Risk Factors
The genetics of schizophrenia suggests the presence of many risk genes, each with small effect sizes (ES). Several recent, large genome-wide association studies (GWAS) have implicated immune genes among the strongest genetic risk factors. Polymorphisms in the major histocompatibility complex (MHC) locus on chromosome 6 are associated with the increased risk of schizophrenia (Shi et al, 2009), and the strongest signal within the MHC is the complement protein C4 locus (Sekar et al, 2016). Another GWAS of ~37 000 patients and 113 000 controls found that immune genes were enriched among 108 conservatively defined loci that met genome-wide significance criteria (Psychiatric Genomics Consortium, 2014).
Prenatal Immune Risk Factors—the Role of Infectious Agents
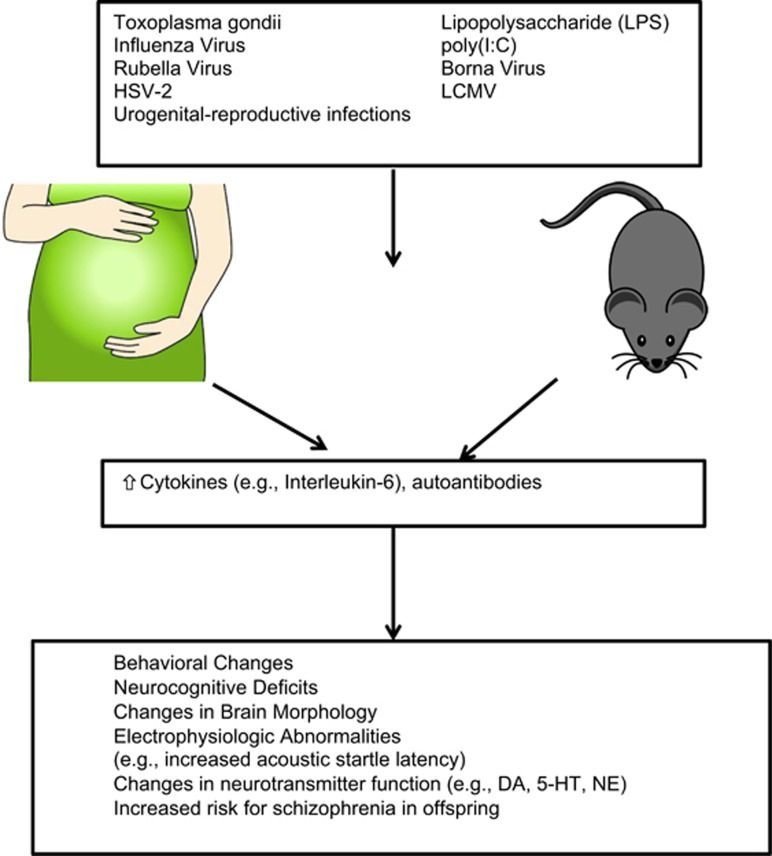
Prenatal maternal infections with a variety of viral and bacterial agents (Brown and Derkits, 2010), and exposure to the parasite Toxoplasma gondii (Torrey et al, 2007), are a replicated risk factor for schizophrenia. Given the myriad of different infectious agents associated with schizophrenia, the inflammatory response to infection has been posited as a potential common mediator of these associations. Animal models of maternal immune activation (MIA) with adjuvants in the absence of a specific pathogen—including synthetic double-stranded RNA, poly(I:C), which induces an anti-viral inflammatory response, or lipopolysaccharide (LPS), which mimics bacterial infection, as well as cytokines (eg, interleukin-6 (IL-6))—have been investigated (Meyer, 2014). In these animal models, changes in behavior, neurocognition, brain morphology, blood cytokine levels, and neurotransmitter function with homology to schizophrenia have been observed in adult offspring. IL-6 and IL-17 appear to drive many of the poly(I:C) MIA model effects on the fetal brain (Choi et al, 2016; Smith et al, 2007). Similarly, IL-1 appears to play a role in neurodevelopmental insults in LPS models (Girard et al, 2010). Furthermore, recent evidence has demonstrated that the effects of MIA may not be mediated solely by inflammatory cytokines, but also epigenetic changes affecting neurotransmitters (Labouesse et al, 2015), and behavioral and cognitive deficits across multiple generations (Weber-Stadlbauer et al, 2016). In animal models, prenatal viral infections can alter monoamine systems, including increased serotonin and norepinephrine levels in the frontal cortex (Pletnikov et al, 2000), as well as DOPAC, the primary metabolite of dopamine (Solbrig et al, 1996).
Non-human primate offspring of mothers infected with a double-stranded RNA virus also shows deficits in communication and social interaction (Bauman et al, 2014). There is parallel evidence in humans that alterations in maternal cytokines during pregnancy are associated with schizophrenia risk. An increase in pro-inflammatory cytokines, including second trimester maternal serum IL-8 (Brown et al, 2004) and maternal serum tumor necrosis factor (TNF-α) in late pregnancy (Buka et al., 2001), have been associated with increased schizophrenia risk, whereas a recent study found that elevated maternal anti-inflammatory cytokines (IL-4, IL-5, and IL-13) during pregnancy conferred a significant decreased risk of schizophrenia (Allswede et al, 2016). Furthermore, inflammation is a potential common mediator of other known pre- and perinatal risk factors for schizophrenia, and prenatal exposure to inflammation is associated with abnormalities in immune function, neurocognition, brain morphology, and gene expression in adult patients with schizophrenia (Miller et al, 2013). These findings support the hypothesis that prenatal inflammation may alter the fetal brain development, thereby increasing the vulnerability to schizophrenia.
In addition to the inflammatory response, maternal infection may lead to other pathophysiological processes that may contribute to an increased risk for schizophrenia in the offspring, including the induction of oxidative stress, as well as deficiencies in micro- and macronutrients (Labouesse et al, 2015; Meyer 2013). Evidence for oxidative stress includes studies demonstrating that pre-treatment of pregnant mice with antioxidants such as N-acetylcysteine (NAC) can reverse the adverse effects of LPS on the fetus, including cytokine induction, hypomyelination, cognitive impairments, and intra-uterine death (Buhimschi et al, 2003; Lanté et al, 2008; Paintlia et al, 2008). Availability of micronutrients such as iron and zinc, which are important for the fetal development, is also affected by maternal infection. Inflammation-induced hypoferrermia is mediated by IL-6 and IL-1β (Lee et al, 2010; Nemeth et al, 2004). Pre-treatment with iron supplementation prevents adverse developmental effects of inflammatory stimuli in an MIA model (Aguilar-Valles et al, 2010). Similarly, maternal zinc deficiency may contribute to developmental abnormalities in offspring via pro-inflammatory cytokine-mediated induction of the zinc-binding protein metallothionein (Vallee and Falchuk, 1993; Coyle et al, 2009).
However, it is important to note that although prenatal maternal infections are a robust risk factor for schizophrenia in the offspring, the majority of exposed persons do not subsequently develop schizophrenia. This observation suggests that the interactions with genetic, epigenetic, or other environmental risk factors may interact to mitigate schizophrenia. Indeed, several studies have reported synergistic actions between maternal infections and genetic risk factors on schizophrenia risk (Børglum et al, 2014; Clarke et al, 2009; Demontis et al, 2011). Furthermore, environmental factors associated with maternal infection (ie, immune-related environment by environment interactions), including maternal anemia (Nielsen et al, 2016; Harvey and Boksa, 2014) and pre-pubertal stress (Giovanoli et al 2013), have been shown to further increase risk for schizophrenia. Figure 1 summarizes the role of various infectious agents in psychosis-like behaviors in laboratory animals and in individuals diagnosed with schizophrenia.
Figure 1.
Summary of the role of various infectious agents in psychosis-like behaviors in laboratory animals and in some patients with schizophrenia.
Premorbid Immune Risk Factors
Immune-based premorbid risk factors for schizophrenia also appear to extend beyond the prenatal period to include the neonatal period, childhood and adolescence. A nested case-control study did not find an association between a panel of inflammatory makers in neonatal dried blood spots and schizophrenia risk (Nielsen et al, 2015). By contrast, lower levels of neonatal acute phase proteins in dried blood spots were associated with the significant increased risk of subsequent schizophrenia and other non-affective psychoses (Gardner et al, 2013). The authors posited that decreased levels of acute phase proteins may increase the susceptibility to infection or adversely affect neurodevelopment.
The association between schizophrenia and immune dysfunction in childhood and adolescence is more robust and appears to be bidirectional: hospital contact for autoimmune disorders and/or infections during childhood or adolescence is associated with an increased risk of schizophrenia in adulthood (Nielsen et al, 2014), and schizophrenia is also a risk factor for autoimmune disorders and infections (Benros et al, 2014). Furthermore, the presence of an autoimmune disorder and hospital contact for infections may act synergistically on subsequent risk of schizophrenia. In a large cohort study, blood IL-6 levels at age 9 were associated with risk of psychotic experiences and fulminant psychotic disorder at age 18 (Khandaker et al, 2014). Furthermore, higher IL-6 levels in childhood were associated with risk of psychotic experiences in a dose-dependent manner. Taken together, these findings implicate the immune system as a risk factor for schizophrenia with effects that may persist into adulthood.
Immune dysfunction in patients with schizophrenia
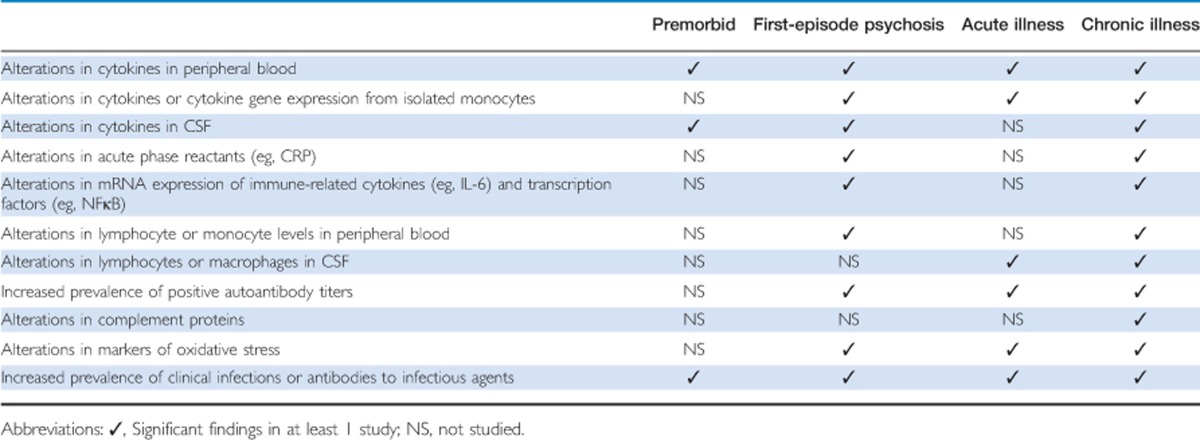
There is evidence that patients with schizophrenia have immune abnormalities in the blood, CSF, and CNS, including immune cell numbers, inflammatory markers, and antibody titers. Inflammation is the complex response to harmful stimuli, and involves activation and recruitment of immune cells, increased blood supply and vascular permeability, and molecular mediators, such as cytokines, C-reactive protein (CRP), complement proteins, antibodies, and reractive oxygen and nitrogen species. Two important themes emerge from these studies. First, many immune abnormalities are present in subjects with first-episode psychosis (FEP) compared with controls, suggesting an association that may be independent of the effects of antipsychotic medications. Second, the concentrations of some immune markers may vary with the clinical status of patients: in other words, there appear to be separate groups of state and trait markers. Evidence for immune dysfunction across the clinical course in schizophrenia is summarized in Table 1, and is reviewed in greater detailbelow.
Table 1. Summary of Evidence for Immune Dysfunction Across the Clinical Course in Schizophrenia.
Cytokines
Cytokines are key signaling molecules of the immune system that exert effects in the periphery and brain. They are produced by immune and non-immune cells, and exert their effects by binding specific receptors on a variety of target cells. Cytokine receptors also exist in soluble forms, which can inhibit (eg, soluble IL-2 receptor (sIL-2 R)) or enhance (eg, sIL-6 R)) the biological activity of cytokines. There are also endogenous cytokine receptor antagonists (eg, IL-1 receptor antagonist (IL-1RA)), which compete with cytokines for membrane receptors. Cytokines are key regulators of acute and chronic inflammation, a complex but vital biological response that impacts all organ systems. Cytokines help coordinate the function of both the innate and adaptive components of the immune system, as well as a host of other physiological processes throughout the body (Florencio-Silva et al, 2015; Ingman and Robertson, 2009).
In a meta-analysis of 40 studies, Miller et al (2011) found that blood cytokine alterations were similar in magnitude and direction for patients with FEP and in patients with acute exacerbation of chronic schizophrenia compared with controls. Patients with schizophrenia had higher blood levels of IL-1β, IL-6, and transforming growth factor-β (TGF-β) during an exacerbation of symptoms, but levels significantly decreased with antipsychotic treatment, suggesting that these are potential state markers for acute illness. By contrast, blood levels of IL-12, interferon- γ (IFN-γ), TNF-α, and sIL-2 R appeared to be trait markers, as levels were higher in patients with FEP and in patients with chronic illness, during both periods of symptomatic worsening and during periods of clinical stability, than in controls. Another meta-analysis of 23 studies of medication naive FEP also found significant elevations in blood levels of IL-1β, IL-6, sIL-2 R, and TNF-α, but not IFN-γ compared with controls (Upthegrove et al, 2014). CSF cytokine levels have been less frequently studied. In a meta-analysis of 16 studies, levels of IL-1β, IL-6, and IL-8 were significantly increased, and levels of sIL-2 R significantly decreased in patients with schizophrenia compared with controls (Wang and Miller, 2016). A recent, study found evidence of increased mRNA levels of immune-related cytokines (including IL-6) and transcription factors (including nuclear factor-κB) in the prefrontal cortex of patients with schizophrenia compared with healthy subjects, and in a poly(I:C) model there was evidence that the observed alterations may be attributable to adult, but not prenatal, immune activation (Volk et al, 2015). In contrast, other animal model studies have found evidence for persistent cytokine alterations in the offspring of immune-challenged mothers (Gary et al, 2013; Giovanoli et al, 2016).
Cytokine abnormalities are not specific to schizophrenia. In addition to schizophrenia, bipolar disorder and major depressive disorder (MDD) have also been associated with aberrant blood cytokine levels. A meta-analysis of blood cytokines in acutely ill patients (68 studies) and chronically ill patients (40 studies) with these three major psychiatric disorders was recently performed (Goldsmith et al, 2016). Levels of two cytokines (IL-6 and TNF-α), one soluble cytokine receptor (sIL-2 R), and one cytokine receptor antagonist (IL-1RA) were significantly increased in acutely ill patients with schizophrenia, bipolar mania, and MDD compared with controls, and levels of these cytokines significantly changed following treatment of the acute illness. In chronically ill patients, levels of IL-6 were significantly increased in schizophrenia, euthymic (but not depressed) bipolar disorder, and MDD compared with controls. Levels of IL-1β and sIL-2 R were also significantly increased in both chronic schizophrenia and euthymic bipolar disorder. The overall similarities in the pattern of cytokine alterations in schizophrenia, bipolar disorder, and MDD during acute and chronic phases of illness, raises the possibility of common underlying pathways for immune dysfunction.
Cytokine alterations in other relevant populations are less well characterized, including prodromal psychosis and treatment-resistant schizophrenia. Two studies found increased blood IL-6 levels in subjects with at-risk mental state (ARMS; Stojanovic et al, 2014) and ultra-high risk (UHR) psychosis (Zeni-Graiff et al, 2016). There are no replicated for any other blood cytokines in the psychosis prodrome. Hayes et al (2014) found that CSF IL-6 R, TGF-α, and TNF-R2 were significantly decreased, and IL-8 levels significantly increased in subjects with ARMS compared with healthy controls. In the North American Prodrome Longitudinal Study project, a panel of 15 blood analytes—which included IL-1β and IL-7—distinguished subjects with clinical high-risk symptoms who developed psychosis from subjects, who did not develop psychosis over a 2-year follow-up period and unaffected controls (Perkins et al, 2015).
Several studies have explored cytokine alterations in (generally) small samples of patients with treatment-resistant schizophrenia. There is evidence for significant higher IL-1β and IL-6 levels in clozapine-treated patients vs controls in at least two studies, although there are failures to replicate (Chen et al, 2008; Haack et al, 1999; Klemettila et al, 2014; O'Connell et al, 2014; Schmitt et al, 2005). In longitudinal studies of patients before and after initiation of clozapine, there is evidence that treatment was associated with significant increases in sIL-2 R, and IL-6 in at least two studies, although there are also failures to replicate. The effects of clozapine on TNF-α are inconsistent (Ajami et al, 2014; Kluge et al, 2009; Löffler et al, 2010a, 2010b; Maes et al, 1997; Monteleone et al, 1997; Pollmächer et al, 1996). Other immunomodulatory effects of clozapine have been reviewed elsewhere (Røge et al, 2012).
Given that schizophrenia is associated with aberrant levels of inflammatory markers and that polymorphisms in cytokines genes may impact not only schizophrenia risk, but also blood cytokine levels, several recent meta-analyses have also investigated associated polymorphisms (Gao et al, 2014; Hudson and Miller, 2016; Qin et al, 2013; Shibuya et al, 2014; Xu et al, 2010). Associations with schizophrenia risk have been found for the genes for IL1B, IL6, sIL6R, and IL-10, but not IL-2, IL-4, TGF-β1, or TNF-α. These polymorphisms are relevant to future studies of immune markers in schizophrenia.
C-Reactive Protein
CRP is an acute phase protein—plasma proteins synthesized by the liver in response to inflammation—that is induced by cytokines, particularly IL-6. In a meta-analysis of eight studies, there was a 28% prevalence of an elevated blood CRP level (defined as >5 mg/l) in patients with schizophrenia and related disorders (Miller et al, 2014). Fernandes et al (2016) performed a meta-analysis of 26 cross-sectional or longitudinal studies of CRP. They found a medium-to-large ES (ES=0.60) for significantly higher blood CRP in patients with schizophrenia vs controls. The ES was similar in studies of patients with FEP. Furthermore, there were no significant changes in blood CRP levels after initiation of antipsychotic medication, suggesting that elevated CRP is a trait marker for schizophrenia. In meta-regression analyses, the association between schizophrenia and CRP was moderated by higher body mass index (BMI), younger age, and higher positive symptom scores.
Labad et al (2015) found that blood CRP levels were non-significantly higher in ARMS subjects who did not transition to a psychotic disorder, and non-significantly lower in ARMS subjects who did transition to psychosis compared to healthy controls. Three previous studies have reported on CRP levels in patients with treatment-resistant schizophrenia, and findings suggesting that there is a transient increase in CRP with initiation of clozapine (Carrizo et al, 2008; Klemettila et al, 2014; Löffler et al, 2010a, 2010b).
Immune Cells
Peripheral leukocytes play important roles in both the innate (eg, granulocytes, monocytes/macrophages, and natural killer cells) and adaptive (eg, B and T lymphocytes) arms of the immune system, and are important sources of cytokines. In a meta-analysis of 16 studies of blood lymphocytes (absolute levels and/or proportions), there was a significant increase in the CD4% and CD56% in acutely ill patients with schizophrenia compared with controls (Miller et al, 2013). Absolute levels of total lymphocytes, CD3 cells, CD4 cells, and the CD4/CD8 ratio were significantly increased, and the CD3% was significantly decreased in patients with FEP vs controls. In addition, the CD4/CD8 ratio significantly decreased, and absolute CD56 levels increased following antipsychotic treatment for acute psychosis.
A meta-analysis of the blood mononuclear phagocytic system has not been performed owing to a small number of studies; however, there is evidence that it may play a role in the pathophysiology of schizophrenia in some patients. Several studies have reported alterations in cytokine secretion or production from isolated blood monocytes (in both unstimulated and stimulated conditions) in patients with schizophrenia compared with controls (Kowalski et al, 2001; Krause et al, 2012a; Krause et al, 2012b; Müller et al, 2012). Drexhage et al (2010) found evidence of a pro-inflammatory gene expression signature in monocytes of patients with recent-onset schizophrenia. Furthermore, a retrospective analysis of serial blood samples found that monocytosis was associated with a worsening of psychotic symptoms, and in some patients the monocytosis resolved with a change in antipsychotic treatment during hospitalization (Dimitrov, 2011). There is also evidence for immune cell abnormalities in the CSF. One group found a significantly increased proportion of CSF macrophages and activated lymphocytes (Nikkila et al, 2001), and also a significantly increased proportion of CSF macrophages in patients with acute psychosis compared with controls (Nikkila et al, 1999). Evidence from postmortem studies also supports a role for neuroinflammation/microglial activation in schizophrenia (Steiner et al, 2006; Steiner et al, 2008).
Antibodies, Autoantibodies, and Comorbid Autoimmune Disorders
Serologic studies have found associations between maternal antibodies to a variety of infectious agents and risk of schizophrenia in the offspring (Brown and Derkits, 2010).
There is an increased prevalence of multiple autoimmune diseases in patients with schizophrenia (Benros et al, 2011; Chen et al, 2012; Eaton et al, 2006), non-affective (Eaton et al, 2010) and affective (Gilvarry et al, 1996) psychoses, as well as their first-degree relatives (Eaton et al, 2006; Eaton et al, 2010; Gilvarry et al, 1996) compared with controls. Specific autoimmune disorders with an increased prevalence in both patients with schizophrenia and their first-degree relatives include celiac disease, Sjogren's syndrome, thyrotoxicosis, interstitial cystitis, and acquired hemolytic anemia (Eaton et al, 2006; Eaton et al, 2010). The association between schizophrenia and autoimmune disorders appears to be bidirectional. Both a personal (Eaton et al, 2006) as well as a family history (Eaton et al, 2010) of any autoimmune disease is also associated with a significant increased risk of schizophrenia. Conversely, patients with schizophrenia have an increased risk of subsequent autoimmune disease, and a history of hospitalization for infection acts synergistically on this risk, and a family history of schizophrenia is associated with a small, but significant increased risk of developing an autoimmune disease (Benros et al, 2014).
In a meta-analysis of 81 studies, there was a significant increased prevalence of positive titers for 20 different autoantibodies in patients with schizophrenia compared with controls (Ezeoke et al, 2013). Absolute titers of anti-cardiolipin IgG and IgM, and nerve growth factor were significantly increased in patients with schizophrenia compared with controls. Lachance and McKenzie (2014) performed a meta-analysis of 12 studies of biomarkers of gluten sensitivity, finding a significant increased odds of non-affective psychosis in subjects with anti-gliadin IgG and IgG, anti-TTG2 IgA, and anti-wheat antibodies, but not anti-EMA IgA, anti-GTTG2 IgG, anti-DGP IgG, and anti-gluten antibodies, suggesting an immune response that differs from patients with celiac disease. Another meta-analysis found an increase in NMDA receptor antibodies (various classes and different receptor subunits) in patients with schizophrenia, bipolar disorder, and MDD, compared with controls based on high- but not low-specificity seropositivity thresholds (Pearlman and Najjar, 2014). There was no difference in the prevalence of these autoantibodies in FEP compared with chronic schizophrenia.
One unresolved question is what factors might contribute to the observed increased prevalence of autoantibodies in schizophrenia? The most frequently posited factors include medication effects, genetics, and age, although none of these have been adequately explored.
Another important question is what is the pathophysiologic significance of positive autoantibodies in patients with schizophrenia, particularly in the absence of other signs and symptoms of autoimmune disease. There is preliminary evidence that some patients with positive NMDA receptor antibodies may have milder, ‘incomplete' forms of NMDA receptor encephalitis with isolated psychotic symptoms in the absence of other neurologic symptoms, and that (open-label) treatment with immunotherapy (eg, corticosteroids or plasmapheresis) may be beneficial for these patients (SIRS Symposium, 2014).
Complement
The study by Sekar et al (2016) of complement C4 (which exists in humans as two different genes, C4A and C4B) has rejuvenated interest in the role of the complement system in patients with schizophrenia. The complement system is involved in innate and adaptive immune responses, consisting of ~35 plasma and cell-surface proteins that recognized foreign or altered host materials. After recognition of foreign molecules, complement proteins opsonize by serving as targets for phagocytes that express complement receptors. Further complement activation results in multi-protein complexes that can lyse target cells. The three pathways of complement activation include classical, alternative, and lectin pathways (Mayilyan et al, 2008).
Two or more groups have reported altered activity of blood C1, C3, and C4, in schizophrenia, although there are failures to replicate (Arakely et al, 2011; Boyajyan et al, 2010; Kano et al, 2011; Kucharska-Mazur et al, 2014; Mayilyan et al, 2008; Severance et al, 2012; Santos Sória et al, 2012). In a proteomic analysis of CSF samples, Jiang et al (2003) found non-significant alterations in complement C3 in patients with schizophrenia versus controls. Sekar et al (2016) found that in postmortem human brain samples, (1) C4A and C4B RNA expression increased in proportion to C4A and C4B copy number, (2) expression levels were two to three times greater for C4A than for C4B, and (3) the copy number of the C4 long form (which contains an insertion of a human endogenous retrovirus) increased the ratio of C4A to C4B expression. Across five brain regions, they found a 1.4-fold greater C4A RNA expression in patients with schizophrenia compared with controls. Given evidence that complement proteins can influence synaptic pruning during critical periods of the brain development, this represents an important future area of research.
Oxidative Stress
Oxidative stress refers to an imbalance of free radicals, such as reactive oxygen and nitrogen species, which are generated from both normal metabolism—including neurotransmitters associated with schizophrenia such as dopamine and glutamate—and from various environmental exposures. The failure of antioxidant defenses to protect against free radical generation damages cell membranes, with resulting dysfunction that may impact on neurotransmission and, ultimately, symptomatology in schizophrenia in some patients (Yao and Keshavan, 2011). Inflammation and oxidative stress reciprocally induce each other in a positive-feedback manner (Bitanihirwe and Woo, 2011). Although the ‘starting point' of inflammatory and oxidative stress abnormalities in schizophrenia remains unclear, several hypotheses have been postulated, including activated microglia (Monji et al, 2009), lower/impaired anti-oxidant defenses (Yao and Keshavan, 2011), developmental redox dysregulation (Do et al, 2009), and impaired glutathione synthesis (Gysin et al, 2007). Decreased CSF concentrations of the antioxidant superoxide dismutase-1 (SOD1), for example, have been found in patients with recent-onset schizophrenia compared with healthy controls, and in fact, this recent-onset group had lower levels then patients with chronic schizophrenia (Coughlin et al, 2013).
In a meta-analysis of 44 studies, Flatow et al (2013) found evidence for abnormal blood markers of oxidative stress in patients with schizophrenia, including FEP. Similar to the pattern observed for cytokines, CRP, and immune cells, evidence was found for state and trait markers of schizophrenia. Total antioxidant status, red blood cell (RBC) catalase and plasma nitrite appeared to be a potential state markers of acute psychosis, as levels were significantly decreased in FEP, and significantly increased following antipsychotic treatment or in clinically stable outpatients. In contrast, RBC SOD appeared to be a trait marker for schizophrenia, as levels were significantly decreased during both periods of symptomatic worsening and during periods of clinical stability.
Consequently, antioxidants including NAC, vitamin C, and vitamin E, have been investigated as adjunctive treatment in schizophrenia. A systematic review of 22 randomized controlled trials of antioxidants found small, but significant reductions in psychopathology over a median 8 weeks of follow-up, although the evidence was deemed low quality (Magalhães et al, 2016). Omega-3 polyunsaturated fatty acids (PUFAs), which may lower levels of free radicals and increase antioxidant enzyme activity, have shown promise as an adjunctive treatment for schizophrenia. A meta-analysis of 10 studies found that supplementation with omega-3 PUFAs reduced symptom severity and decreased conversion rates in subjects with prodromal psychosis; decreased nonpsychotic symptoms and antipsychotic medication dosages, and improved early treatment response rates in FEP; but had mixed results in patients with chronic schizophrenia (Chen et al, 2015). These findings suggest a potential role for omega-3 PUFAs in earlier stages of illness.
Imaging of Neuroinflammation in Schizophrenia
There is robust evidence for neuroinflammation in schizophrenia (Najjar et al, 2013), which has been proposed as a potential mechanism underlying structural and functional brain disconnectivity, with evidence of increased density and activation of microglia, resident immune cells of the brain, at various stages of the illness (Laskaris et al, 2016; Schmitt et al, 2011). In a systematic review of 15 studies, Najjar and Pearlman (2015) found that five neuropathological and two neuroimaging studies collectively yielded consistent evidence of an association between schizophrenia and microglial activation, particularly in white vs gray matter regions. In subjects with schizophrenia, but not in controls, ultrastructural analysis revealed activated microglia near dystrophic and apoptotic oligodendroglia, demyelinating and dysmyelinating axons and swollen and vacuolated astroglia. A neuropathological study found that white matter neuronal density in the orbitofrontal cortex was increased in schizophrenia cases with high transcription levels of pro-inflammatory cytokines relative to both those exhibiting low transcription levels and to controls (Fung et al, 2014). Astrogliosis was consistently absent. In addition, several studies have explored relationships between markers of inflammation in the peripheral blood and brain volumes. There is evidence for an association between blood IL-6 levels and smaller hippocampal volumes in three studies, including in FEP (Kalmady et al, 2014; Miller et al, 2014; Mondelli et al, 2011), although another study failed to replicate this association (Hoseth et al, 2016).
Imaging microglial activation using positron emission tomography (PET) is another approach used to investigate neuroinflammation in schizophrenia (Laskaris et al, 2016). Microglia are the immune cells in the brain, derived from myeloid precursor cells that respond to harmful stimuli by activating the immune system (Ginhoux et al, 2010; Kettenmann et al, 2011). Quiescent, resting state microglia actively survey the brain for injury or inflammation (Nimmerjahn et al, 2005). When stimulated, microglia will rapidly shift morphology into an activated state, migrate to the pathogen site, and release various cytokines and other molecular mediators, including reactive oxygen species, glutamate, insulin-like growth factor 1, brain-derived neurotrophic factor, and cyclooxygenase 1. These molecules simultaneously promote an inflammatory response (M1 phenotype) and downregulate the inflammatory response (M2 phenotype; Réus et al, 2015). Although some postmortem studies have reported increased microglial cell numbers and densities in various brain regions, others have shown no difference in schizophrenia vs control subjects (reviewed in Laskaris et al, 2016). Moreover, recent data suggest that microglia are regulated in a dynamic manner across different temporal stages, each with unique regulatory circuitry. This may be relevant for how and when the immune system interacts with microglia in the brain, thereby mediating schizophrenia risk (Matcovitch-Natan et al, 2016).
In the activated state only, microglia express translocator protein (TSPO), and TSPO radioligands allow for PET imaging of activated microglia (Ching et al, 2012). The literature using TSPO PET ligands remains nascent. Three previous studies have used an (R)-[11C]PK11195 PET ligand in small samples (<25 total subjects). van Berckel et al (2008) found an increase in binding potential in total gray matter in patients with recent-onset (first 5 years) of schizophrenia vs controls. Banati and Hickie (2009) found that in 15 of 28 a priori regions of interest patients with (recent and chronic) schizophrenia had increased binding potential, particularly on the right side of the brain. Doorduin et al (2009) found a higher-binding potential was found in the hippocampus of patients in recovery from psychosis compared with controls.
More recent studies, also with small samples (<30 total subjects), have used second-generation radioligands that have greater binding to TSPO compared with the (R)-[11C]PK11195, have been largely negative. There was no significant difference in binding potential between patients with chronic schizophrenia and controls using [11C]-DAA1106 ligand (Takano et al, 2010). However, in that study overall cortical binding was significantly correlated with positive symptoms and illness duration. Kenk et al (2015) found no difference in [18F]-FEPPA ligand binding between patients with chronic schizophrenia and controls. Using a [11C]DPA-713 ligand, Coughlin et al (2016) found no difference in binding between patients with recent onset schizophrenia and healthy controls; however, patients had significantly increased blood and CSF IL-6. By contrast, Bloomfield et al (2016) found that [11C]-PBR28-binding potential was increased in subjects at UHR for psychosis compared with controls in total, frontal, and temporal gray matter. Furthermore, binding potential was correlated with psychotic symptoms, but not depressive symptoms, in the UHR group. However, positive findings in this study were based on the distribution volume ratio, whereas there were no differences in regional distribution volume, which is the traditional outcome measure in similar PET studies (Narendran and Frankle, 2016). Nevertheless, these methodological issues suggest the need for more uniformity in the field in how to study and interpret studies of imaging neuroinflammation in schizophrenia.
Though small, the literature on TSPO binding in patients with schizophrenia is mixed and at present, the field should likely interpret the results with some reservation, especially given the preponderance of negative findings using second-generation radiotracers, and the dissociation between TSPO binding and other markers of inflammation (Coughlin et al, 2016). There is a suggestion that activated microglia may be more relevant in at-risk and early-phase groups and, might be a marker of disease severity. However, lack of uniformity in radioligands and outcome measures make it challenging to compare results across studies. Furthermore, only the three most recent studies have considered the rs6971 polymorphism in the TSPO gene, which may be an important confound affecting binding (Bloomfield et al, 2016; Coughlin et al, 2016; Kenk et al, 2015). TSPO expression may also be expressed in partially activated microglia, another potential confounding factor (Marshall et al, 2013). Moreover, TSPO is expressed in other non-microgial cell types, including in astrocytes and endothelial cells, even at basal, non-inflammatory levels (Cosenza-Nashat et al, 2009). Future studies may consider the M1 vs M2 activation state in imaging studies of microglia, which may reflect the balance of pro- and anti-inflammatory processes in activated microglia. Finally, an important consideration is the fact that TSPO is a mitochondrial protein. Mitochondrial dysfunction has been described in schizophrenia and other neuropsychiatric disorders, and related alterations in oxidative stress may pay a role in immune pathways relevant for the disorder (Rajasekaran et al, 2015). TSPO expression and binding may likely be influenced by these mitochondrial deficits and as such, this must be considered in the interpretation and future design of TSPO PET studies.
Infections
As indicated above, there is rich preclinical and clinical data indicating a role of maternal infection in the development of schizophrenia. Nevertheless, it should be noted that schizophrenia is associated with increased infections throughout the lifespan. Patients with schizophrenia appear to have an increased prevalence of certain comorbid infections. Several studies have found an increased prevalence of lower urinary tract infections (UTI) in patients with schizophrenia, particularly during episodes of illness exacerbation (Graham et al, 2014; Miller et al, 2013) and this may be a recurrent phenomenon (Laney et al, 2015). In a meta-analysis of 16 independent samples (2353 patients and 1707 controls), there was a significant 1.7-fold increase in risk of positive T. gondii IgM antibodies—a marker of acute/recent exposure or reinfection—in patients acute psychosis compared with controls, and the association was stronger for patients with acute exacerbations of chronic schizophrenia (Monroe et al, 2015). Several studies have also found an increased prevalence of active viral (Ahokas et al, 1987; Krause et al, 2010; Srikanth et al, 1994) and chlamydial (Fellerhoff et al, 2007) infections in hospitalized patients with acute psychosis, although one study failed to replicate the latter association (Park et al, 2012).
An important limitation of these studies is that they do not permit inferences regarding either temporal or causal aspects of the association between acute psychosis and infection. IgM antibodies can remain positive for months after certain infections, and can also re-emerge following reactivation of latent infections. Symptoms including disorganization, delusions, and negative symptoms, and impulsivity may be associated with decreased self-care or other behaviors that could increase risk of certain infections. By contrast, it is possible that some infections may precede episodes of acute psychosis. There is evidence that differential white blood cell counts distinguish patients with vs without UTI, raising the possibility that the inflammatory response to infection may contribute to acute psychosis (Laney et al, 2015; Miller et al, 2013). It has been hypothesized that infection and associated inflammation during critical periods of neurodevelopment may permanently alter the ‘set-point' of the immune system (Bilbo and Schwarz, 2009), which might confer increased susceptibility to infection, or to adverse neuropsychiatric effects from infection, in patients with schizophrenia. Patients with schizophrenia may have reduced neutrophil phagocytosis (McAdams and Leonard, 1993), as well as lower neutrophil bactericidal reserve and a greater incidence of subclinical bacterial infection (Rwegellera et al, 1982). Abdeljaber et al (1994) found a significant decrease in natural killer cell activity—first-line defenders against infections—in patients with schizophrenia.
Schizophrenia is also associated with increased mortality from infectious diseases, including pneumonia and influenza (Saha et al, 2007). A recent study found that elevated levels of Epstein–Barr virus and Herpes Simplex virus type 1 antibodies are also associated with a small but significant increased risk of death from natural causes in patients with schizophrenia (Dickerson et al, 2014). Thus, although multiple factors may contribute, immune dysfunction—manifested by an increased prevalence of certain infections—may contributes to infectious disease comorbidity and mortality in some patients with schizophrenia.
Critical appraisal of the literature on immune dysfunction in schizophrenia
The literature on immune dysfunction in schizophrenia spans decades. However, there is significant heterogeneity in the literature regarding findings, including negative studies even for markers that are well replicated (eg, blood IL-6 levels). Many statistically significant findings for immune markers have small-to-moderate ESs. This suggests that either the observed change is either relatively minor and may not be clinically significant, or, alternatively, immune disequilibrium occurs in a subset of patients with schizophrenia. That baseline blood levels of cytokines predicted response to adjunctive NSAID treatment in some studies (Laan et al, 2010; Muller et al, 2004) is one piece of evidence suggesting that the latter hypothesis warrants further investigation.
An intriguing paper (Manu et al, 2014) evaluated the evidence for inflammation and schizophrenia through the prism of the Bradford Hill criteria, these criteria include a number of elements that are italicized below, and together represent an epidemiological tool for evaluating the evidence for causation. They concluded that there is robust evidence for strength and consistency and modest evidence for plausibility of the association between inflammation and schizophrenia, but evidence is lacking for a biological gradient and temporality. Importantly, they noted the association lacks specificity because inflammation is also associated with other major psychiatric disorders (eg, bipolar disorder and MDD). This framework has broader applicability to all aspects of immune dysfunction in schizophrenia. We suggest that several important limitations in this literature; however, preclude definitive conclusions regarding a potential causal association between immune dysfunction and schizophrenia. Addressing these limitations represent important directions for future research.
First of all, findings for individual immune markers should be interpreted with caution in light of relatively small number of studies and small cumulative sample size available for many comparisons. For many markers, we cannot account for either the type or the length of pharmacologic intervention employed in the treatment trials. Important potential confounding/moderating factors such as race, smoking, BMI, socioeconomic status, insomnia, fasting status and dietary factors, assay methodology, level and type of psychopathology, and genetic heterogeneity cannot be accounted for and/or have been inadequately considered (Koola, 2016; Miller et al, 2011; O'Connor et al, 2009). A minority of studies compared associations between immune markers and demographics, symptoms, and cognition, which impair our ability to make inferences regarding the potential influence of immune dysfunction on schizophrenia psychopathology. In general, studies to adequately compare markers across the clinical course of the disorder (eg, prodrome, first-episode psychosis, acutely ill, clinically stable, treatment-resistant psychosis) are lacking. The majority of studies have focused on blood markers, with a relative paucity of studies that have focused on CSF or postmortem alterations, imaging of neuroinflammation, and so on, as well as correlations between peripheral and central markers of immune dysfunction. Furthermore, few previous studies have stratified patients with and without abnormal immune markers, to investigate if patients with immune dysfunction have a distinct demographic or clinical profile. Such an approach will be essential to evaluating the hypothesis of an immunophenotype of schizophrenia.
Several selection biases may also impact our interpretation of the literature. Some studies that would have otherwise been included in previous meta-analyses of immune markers in schizophrenia were excluded because the necessary summary data were not available, and their influence on results is uncertain. The vast majority of studies excluded recent illicit substance use, and about half of patients with schizophrenia have a lifetime diagnosis of a substance use disorder. As illicit drug use can modulate immune function (Benito et al, 2008; Massi et al, 2006; Volpe et al, 2014; Yang et al, 2015), its effects in schizophrenia are largely unknown.
The evidence to date raises many important questions. What are the most relevant patient populations? What are the most useful set of immune markers to measure? Should we preferentially measure immune markers, where there is more robust evidence (in order to replicate findings), those markers that have not been thoroughly investigated, or some combination of both? Is measurement of peripheral levels of immune markers sufficient (vs flow cytometry of specific leukocyte populations or coupling with CSF analysis and/or brain imaging)? Are demographic and clinical (eg, psychopathology, cognition, and a history of psychosis) features are associated with immune changes? Given evidence for immune changes in other psychiatric disorders that may present with psychosis (eg, bipolar disorder and MDD), is there evidence for a broader immunophenotype of psychosis, just as the B-SNIP study found evidence for psychosis biotypes that did not respect DSM clinical symptom-based diagnostic categories (Clementz et al, 2015).
Despite these many uncertainties, several studies have provided stronger evidence for a potential causal association between immune dysfunction and schizophrenia. For example, the study by Sekar et al (2016) provides compelling evidence that C4 gene may increase schizophrenia risk by influencing synaptic pruning during critical periods of the brain development. In mice, a single maternal injection of IL-6 during pregnancy caused prepulse and latent inhibition deficits in the adult offspring, suggesting the immune response may mediate robust epidemiologic evidence for the association between prenatal maternal infections and schizophrenia risk in the offspring (Smith et al, 2007). Successful treatment with immunotherapy for patients with positive NMDA receptor antibodies and isolated psychotic syndrome raises the possibility of a causal role for these antibodies in affected subjects.
Underlying mechanisms
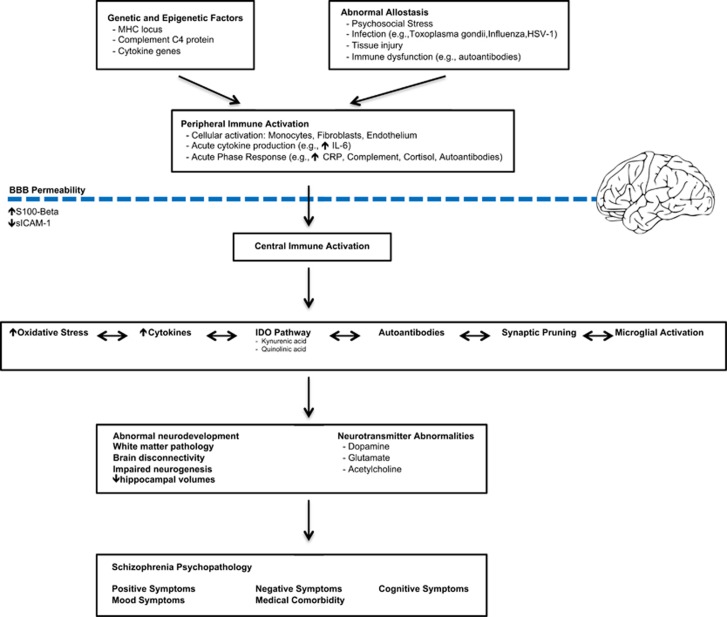
In order to summarize the studies reviewed above, Figure 2 presents a theoretical framework that attempts to integrate findings and relate them to potential mechanisms, whereby immune system dysfunction might mediate illness risk and pathophysiology in some patients with schizophrenia. This model is not intended to be comprehensive, but rather to serve as a catalyst for critical thinking and to suggest potential areas for future research. The model is also intended to have broad applicability, in that these pathways may be relevant to both premorbid factors that influence neurodevelopment—and may permanently alter the ‘set-point' of the immune system—and subsequent schizophrenia risk, as well as factors that influence disease pathophysiology in some patients with schizophrenia. In brief, abnormal allostasis interacts with genetic and/or epigenetic factors, which results in peripheral immune activation, characterized by cellular activation, acute cytokine production, and an acute phase response. Certain genetic factors (eg, complement C4) may directly influence synaptic pruning during critical periods of brain development, and other factors may directly impact the CNS via microglial activation. In the setting of increased blood–brain barrier permeability, there is also central immune activation, including cytokine production, oxidative stress, autoantibodies, tryptophan catabolites (indoleamine 2,3-dioxygenase (IDO) pathway), and microglial activation. Together, these immune factors can directly or indirectly impact on a variety of processes, including abnormal neurodevelopment, white matter pathology, brain disconnectivity, impaired neurogenesis, and neurotransmitter abnormalities, thereby contributing to schizophrenia psychopathology. For example, pro-inflammatory cytokines can directly modulate neurotransmitter function, as systemic increases in serum IL-6 in adult rodents modulate dopamine turnover and sensitization to amphetamine-induced locomotion (Zalcman et al, 1994; Zalcman et al, 1999). By contrast, IDO, the rate-limiting enzyme in tryptophan catabolism, is also expressed in astrocytes and microglia, and its activity can be modulated by cytokines. IDO induction results in increased production of kynurenine, which is converted in astrocytes to the NMDA receptor antagonist kynurenic acid (KYN-A). NMDA receptor hypofunction has been implicated in the pathophysiology of schizophrenia (Gaspar et al, 2009; Martin et al, 2004). Previous studies have found increased blood (Chiappelli et al, 2014; Ravikumar et al, 2000), CSF (Erhardt et al, 2001; Kegel et al, 2014; Nilsson et al, 2005; Schwieler et al, 2015), and postmortem brain (Sathyasaikumar et al, 2011; Schwarcz et al, 2001) levels of KYN-A, as well as increased IDO activity (Barry et al, 2009) in patients with schizophrenia.
Figure 2.
Summary of potential mechanisms whereby immune system dysfunction might mediate illness risk and pathophysiology in some patients with schizophrenia.
More specifically, the immune system may play a role in specific symptom domains, including negative and cognitive symptoms. Inflammation may contribute to pathophysiologic pathways that lead to negative symptoms, namely, decreased motivation and reward processing deficits. Functional neuroimaging studies using reward-based tasks consistently implicate the ventral striatum in reward processing (Salamone and Correa, 2012), which is abnormal in patients with schizophrenia (Juckel et al, 2012; Juckel et al, 2006; Nielsen et al, 2012), neural activity in ventral striatal regions is altered following administration of several inflammatory stimuli including IFN-α, typhoid vaccination, and endotoxin in psychiatrically healthy individuals (Brydon et al, 2008; Capuron et al, 2012; Eisenberger et al, 2010; Harrison et al, 2015). In addition, inflammation mediates deficits in objective assessments of motivation as reflected by effort expenditure in laboratory animals, including non-human primates (Felger et al, 2007; Salamone et al, 1994; Vichaya et al, 2014). This mechanism for motivational deficits has been demonstrated in individuals with MDD and elevated CRP (Felger et al, 2015). Similar mechanisms may contribute to motivational deficits in schizophrenia.
Effects of inflammation on the dorsal striatum leading to psychomotor retardation, as seen laboratory animals (eg, IL-6 or LPS; Frenois et al, 2007; Lenczowski et al, 1999) and in healthy controls given inflammatory stimuli (eg, IFN-α, endotoxin, typhoid vaccination; Haroon et al, 2015; Majer et al, 2008; Raison et al, 2010), may also lead to psychomotor slowing in schizophrenia. Infectious agents may also contribute to psychomotor retardation in schizophrenia. Infected rodents and seropositive humans with T. gondii show psychomotor slowing (Havlicek et al, 2001; Hutchison et al, 1980; Witting, 1979). Patients with schizophrenia and seropositivity to T. gondii also show latency in neural processing speed in an acoustic startle paradigm (Pearce et al, 2013). Toxoplasma could exert its effects on psychomotor slowing via several potential mechanisms. T. gondii contains two tyrosine hydroxylase genes, the rate-limiting enzyme in dopamine synthesis, and thus may increase dopamine synthesis (Gaskell et al, 2009; Webster and McConkey, 2010). T. gondii also requires tryptophan for replication, thereby inducing the IDO pathway and increased KYN-A. Administration of the KYN-A precursor kynurenine to rats in the embryonic to postnatal period increases KYN-A levels through adulthood, reduces alpha-7 nicotinic receptor and metabotropic glutamatergic receptor mRNA expression through adulthood, decreases dendritic spine density, and decreases glutamate release (Pershing et al, 2015). Moreover, these rats have shown cognitive deficits in attentional set shifting (Alexander et al, 2013), as well as in spatial working memory tasks (Pocivavsek et al, 2012). In schizophrenia, there is a single-nucleotide polymorphism in the kynurenine 3-monooxygenase gene that is associated with visuospatial working memory deficits and impairments in smooth pursuit eye movement (Aoyama et al, 2006; Wonodi et al, 2011; Wonodi et al, 2014). These hypothetical mechanisms for specific symptom domains in schizophrenia should be considered in future investigation.
Translational implications: clinical trials of anti-inflammatory agents/Immunotherapy
Two recent meta-analyses have explored the efficacy of adjunctive anti-inflammatory agents in schizophrenia. Nitta et al (2013) analyzed eight studies (n=774 patients) of adjunctive NSAIDs (six trials of celecoxib and two trials of aspirin), including three unpublished reports. They found that NSAIDs were associated with a small reduction in PANSS total score at the trend level (ES=−0.24), and a small, significant reduction in PANSS positive subscale score (ES=−0.19). Importantly, they also found that significant superiority of NSAIDs over placebo for PANSS total scores was moderated by treatment with aspirin, inpatient and first-episode status. That inpatient and first-episode status moderated the efficacy of adjunctive NSAIDs in this meta-analysis is broadly consistent with findings of associations between abnormal immune markers and clinical status. In another meta-analysis of 26 double-blind trials of various anti-inflammatory agents, Sommer et al (2014) found significant effects for aspirin, estrogens, and NAC, but not celecoxib, davunetide, fatty acids, and minocycline. A recent investigation of D-serine in individuals at clinical high risk for psychosis demonstrated benefit in the treatment of negative symptoms in this high-risk population (Kantrowitz et al, 2015).
Studies of other immunomodulatory treatments in schizophrenia have been very limited. Levine et al (1997) reported a trial of adjunctive treatment with the immunosuppressant azathioprine in two parallel groups with treatment-resistant schizophrenia and high titers of anti-platelet autoantibodies. Of the eleven patients, two had significant improvements in symptoms over 7 weeks, and seven had significant reductions in the level of anti-platelet autoantibodies. A recent protocol for a 12-week trial of adjunctive methotrexate in schizophrenia has also been described (Chaudry et al, 2015).
To date, two small studies of cytokine-based immunotherapy in schizophrenia have been published, although several other larger trials are ongoing. A case series of two patients with treatment-resistant paranoid schizophrenia had significant improvement in total psychopathology during open-label adjunctive treatment with recombinant human IFN-γ-1b subcutaneously weekly for 4 weeks, and then tapered down over 2–3 weeks (Grüber et al, 2014). The other study was an 8-week open-label trial of adjunctive tocilizumab (an anti-IL-6 receptor monoclonal antibody), given intravenously at baseline at 4 weeks, in six patients (Miller et al, 2016). Compared with baseline, there was significant improvement in verbal fluency, digit symbol coding, and global cognition at 4 and 8 weeks.
Future research directions
Despite the multitude of preclinical and clinical data suggesting that the immune system may play a role in schizophrenia, the significance of these findings to the development and ultimately the treatment of the disorder remains unclear due to the multiple issues outlined above. Therefore, we highlight below important considerations to guide future human research on immune function in schizophrenia and related disorders. As detailed in Table 1, studies are lacking for a number of immune parameters across the clinical course of illness, particularly in subjects with prodromal or high-risk psychosis. Table 2 outlines the following proposed directions for future investigations of the role of the immune system in schizophrenia psychopathology. Studies should be rigorously designed to match subjects (ie, patients and controls) and/or statistically controls for potential confounding/moderating factors such as age, sex, race, smoking, BMI, socioeconomic status, insomnia, fasting status and dietary factors, assay methodology, level and type of psychopathology, and genetic heterogeneity known to influence immune function.
Table 2. Proposed Directions for Future Investigations of the Role of the Immune System in Schizophrenia Psychopathology.
| Methodological concerns/study design |
| Matching samples on potential confounding/moderating factors |
| Longitudinal study design |
| Birth cohort study design |
| Understudied populations: at-risk mental state, prodromal, first-episode drug naive, treatment-resistant psychosis, relatives of patients with schizophrenia |
| Is there a psychosis immunophenotype that cuts across clinical diagnoses? |
| Does immune response to infections/trauma increase risk for relapse? Would preventative measures decrease risk? |
| Consideration for immune effects of omega-3 fatty acids and NSAIDs |
| Patients with immune dysfunction as an inclusion criteria for trials of anti-inflammatory medications |
| Studying the role of the immune system in acutely ill vs clinically stable patients |
| Trials of adjunctive monoclonal antibody immunotherapy |
| More trials of CSF, postmortem alterations, and imaging of neuroinflammation |
| Concurrent measurement of multiple immune parameters (eg, cytokines, CRP, oxidative stress, and so on) |
| Measurement of overall patterns of immune activation (eg, flow cytometry of macrophages/monocytes vs TH1 vs TH2 vs TH17 cells) |
| Do subjects with schizophrenia and specific patterns of immune dysfunction (eg, anti-NMDA receptor antibodies) have a distinct clinical or demographic profile? |
A complementary approach that might minimize some potential confounds would be to design longitudinal studies with serial measurement of intra-individual changes in immune markers across the clinical course of the disorder. This would also inform whether the association between schizophrenia and immune dysfunction has the property of temporality. Furthermore, this approach can inform treatment effectiveness, advance relapse prevention efforts, and investigate effects of disease progression.
Future studies should investigate associations between immune markers and demographics, symptoms, and cognition in patients. Moreover, more studies are needed to compare markers across the clinical course of the disorder (eg, prodrome, FEP, acutely ill, clinically stable, treatment-resistant psychosis). Birth cohort studies may also offer a unique opportunity to assess relationships between prenatal immune risk factors and immune function in adult patients with schizophrenia. Relatively, fewer studies have been conducted in subjects with prodromal and treatment-resistant psychosis. Studies in subjects with ARMSs would be informative about the potential roles of immune function in transition to psychosis, and might inform potential diagnostic tests and preventive interventions. Studies in drug-naive FEP would help further disentangle associations that are independent of psychotropic medications. Studies in treatment-resistant psychosis might offer new leads in treatment strategies for this patient population, particularly if there is evidence for a different immune profile in these subjects. Furthermore, several studies found that first-degree relatives of people with schizophrenia have abnormal immune function (Gaughran et al, 2002; Liu et al, 2010; Martinez-Gras et al, 2012; Nunes et al, 2006.) It would advance the field to test the hypothesis that relatives have abnormal concentrations of trait markers for schizophrenia.
Given similarities in cytokine alterations across schizophrenia, bipolar disorder, MDD, is there evidence for a immunophenotype of psychosis that cuts across clinical phenomenology? In particular, previous studies of immune function in mood disorders have not stratified subjects by the presence or absence of psychotic features.
Does the immune response to infections increase the risk of relapse in schizophrenia? If confirmed by longitudinal studies with serial measurement of markers over the clinical course, do preventive measures (eg, antibiotic prophylaxis for recurrent infections) decrease that risk?
In terms of treatment, the immune effects of omega-3 fatty acids preventing conversion to psychosis in high-risk subjects, particularly those with evidence of immune dysfunction, such as inflammation or oxidative stress—should be investigated. A trial of NSAIDs for prevention of psychotic disorders should also be considered. The response to immunosuppression or antipsychotic treatment in patients with positive neuronal cell-surface (eg, NMDA receptor) antibodies should also be investigated.
Several strategies may increase the signal-to-noise ratio for adjunctive trials of anti-inflammatory agents. Patients with evidence of inflammation or other immune dysfunction in the peripheral blood may be more likely to respond to an anti-inflammatory treatment strategy than those without inflammation, and should be an inclusion criterion for many future trials. It would also be informative to study acutely ill and clinically stable patients in separate trials, given potential differences in state versus trait markers.
Furthermore, trials of adjunctive monoclonal antibody immunotherapy in schizophrenia are warranted. There are two major potential advantages of monoclonal antibody immunotherapy over NSAIDs or other anti-inflammatory agents. Perhaps most importantly, NSAIDs have relevant off-target (ie, non-immune) effects. By contrast, monoclonal antibodies do not have any off-target effects, only acting on specific inflammatory cytokines. Therefore, improvements in psychopathology in response to monoclonal antibody immunotherapy would further (and directly) implicate inflammatory pathways in the pathophysiology of schizophrenia. Compared with other anti-inflammatory agents, monoclonal antibodies also have more potent anti-inflammatory properties. Indeed, NSAIDs have minimal efficacy in conditions with significant inflammation (eg, autoimmune disorders). In schizophrenia, adjunctive anti-inflammatory agents have been associated with small-to-moderate ES for improvements in psychopathology. Although this most likely reflects that fact that immune system dysfunction occurs in only a subset of patients with schizophrenia, another possibility is that more potent anti-inflammatory agents are needed for more robust effects. This may also help explain why treatment studies that have targeted inflammation in schizophrenia have yielded mixed results.
The majority of previous studies have focused on blood markers, with a relative paucity of studies that have focused on CSF or postmortem alterations, imaging of neuroinflammation, and so on, as well as correlations between peripheral and central markers of immune dysfunction. Such studies will provide valuable clues regarding potential mechanisms of these associations, whether alterations observed in the periphery are mirrored in the CNS across the clinical course of schizophrenia, including effects of treatment.
Concurrent measurement of multiple parameters (eg, cytokines, CRP, oxidative stress, flow cytometry for immune cells, antibodies to infectious agents, and autoantibodies) would increase the ability to make broader inferences regarding immune function. Furthermore, if groups or clusters of covarying markers could be defined, investigation of these markers is likely to yield better signal-to-noise ratios than individual markers and should have more biological validity. It may also be less important to measure individual cytokines or other markers, but instead measure overall patterns of immune activation, for example, flow cytometry of macrophages/monocytes vs TH1 vs TH2 vs TH17 cells.
In order to test the hypothesis that a subset of patients with schizophrenia may manifest an immunophenotype, proposed studies detailed above should exclude patients without or stratify patients with and without abnormal immune markers, to investigate if patients with immune dysfunction have a distinct demographic or clinical profile. Patients with anti-NMDA receptor antibodies and patients with elevated IL-6 represent two such subgroups that warrant further investigation. If present, a biological gradient for the association between schizophrenia and immune dysfunction may be more easily identified using such an approach. The possible existence of multiple immunophenotypes must also be considered (eg, patients with anti-NMDA receptor antibodies may be distinct from those with other immune abnormalities).
In conclusion, the evidence to date has afforded the field an unparalleled opportunity for further studies of immune function that will lead to greater understanding of the etiopathophysiology of schizophrenia in some patients, towards new potential diagnostics and therapeutics to reduce risk, alleviate symptoms and improve quality of life in both at-risk and established patient populations.
Funding and disclosure
Dr Miller has nothing to disclose for this study. In the past 12 months, Dr Miller received research support from the National Institute of Mental Health, American Psychiatric Association, Stanley Medical Research Institute, NARSAD, and Augusta University and Honoraria from Psychiatric Times. Dr Goldsmith has nothing to disclose relevant to the present work. In the past 12 months, Dr Goldsmith received training support from the National Institute of Mental Health (R25MH101079).
References
- Abdeljaber MH, Nair MP, Schork MA, Schwartz SA (1994). Depressed natural killer cell activity in schizophrenic patients. Immunol Invest 23: 259–268. [DOI] [PubMed] [Google Scholar]
- Ahokas A, Rimón R, Koskiniemi M, Vaheri A, Julkunen I, Sarna S (1987). Viral antibodies and interferon in acute psychiatric disorders. J Clin Psychiatry 48: 194–196. [PubMed] [Google Scholar]
- Ajami A, Abedian F, Hamzeh Hosseini S, Akbarian E, Alizadeh-Navaei R, Taghipour M (2014). Serum TNF-α, IL-10 and IL-2 in schizophrenic patients before and after treatment with risperidone and clozapine. Iran J Immunol 11: 200–209. [PubMed] [Google Scholar]
- Alexander KS, Pocivavsek A, Qu HQ, Pershing ML, Schwarcz R, Bruno JP (2013). Early developmental elevations in brain kynurenic acid impair cognitive flexibility in adults: reversal with galantamine. Neuroscience 238: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allswede DM, Buka SL, Yolken RH, Torrey EF, Cannon TD (2016). Elevated maternal cytokine levels at birth and risk for psychosis in adult offspring. Schizophr Res S0920-9964: 30077–30079. [DOI] [PubMed] [Google Scholar]
- Aoyama N, Takahashi N, Saito S, Maeno N, Ishihara R, Ji X et al (2006). Association study between kynurenine 3-monooxygenase gene and schizophrenia in the Japanese population. Genes Brain Behav 5: 364–368. [DOI] [PubMed] [Google Scholar]
- Arakelyan A, Zakharyan R, Khoyetsyan A, Poghosyan D, Aroutiounian R, Mrazek F et al (2011). Functional characterization of the complement receptor type 1 and its circulating ligands in patients with schizophrenia. BMC Clin Pathol 11: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Valles A, Flores C, Luheshi GN (2010). Prenatal inflammation-induced hypoferremia alters dopamine function in the adult offspring in rat: relevance for schizophrenia. PLoS One 5: e10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati R, Hickie IB (2009). Therapeutic signposts: using biomarkers to guide better treatment of schizophrenia and other psychotic disorders. Med J Aust 190: S26. [DOI] [PubMed] [Google Scholar]
- Barry S, Clarke G, Scully P, Dinan TG (2009). Kynurenine pathway in psychosis: evidence of increased tryptophan degradation. J Psychopharmacol 23: 287–294. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Iosif A, Smith SE, Bregere C, Amaral DG, Patterson PH (2014). Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol Psychiatry 75: 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C, Tolón RM, Pazos MR, Núñez E, Castillo AI, Romero J (2008). Cannabinoid CB2 receptors in human brain inflammation. Br J Pharmacol 153: 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benros** ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB (2011). Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry 168: 1303–1310 Nationwide population-based register study which found that autoimmune disorders and severe infections are associated with schizophrenia risk, have interactive effects, and these associations appear to be bidirectional.. [DOI] [PubMed] [Google Scholar]
- Benros ME, Pedersen MG, Rasmussen H, Eaton WW, Nordentoft M, Mortensen PB (2014). A Nationwide study on the risk of autoimmune diseases in individuals with a personal or a family history of schizophrenia and related psychosis. Am J Psychiatry 171: 218–226. [DOI] [PubMed] [Google Scholar]
- Bilbo** SD, Schwarz JM (2009). Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci 3: 14 Review of the evidence that infection during the perinatal period of life acts as a vulnerability factor for later-life alterations in cytokine production, and marked changes in cognitive and affective behaviors throughout the lifespan.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Woo TU (2011). Oxidative stress in schizophrenia: an integrated approach. Neurosci Biobehav Rev 35: 878–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield** PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR et al (2016). Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [(11)c]pbr28 pet brain imaging study. Am J Psychiatry 173: 44–52 Microglial activity is elevated in persons with subclinical symptoms who are at ultra high risk of psychosis and is related to at-risk symptom severity.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyajyan A, Khoyetsyan A, Chavushyan A (2010). Alternative complement pathway in schizophrenia. Neurochem Res 35: 894–898. [DOI] [PubMed] [Google Scholar]
- Børglum AD, Demontis D, Grove J, Pallesen J, Hollegaard MV, Pedersen CB et al (2014). Genome-wide study of association and interaction with maternal cytomegalovirus infection suggests new schizophrenia loci. Mol Psychiatry 19: 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ (2010). Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 167: 261–280 Critical evaluation of the epidemiologic literature on in utero exposure to infection and schizophrenia, with discussion of putative unique and common mechanisms by which in utero exposure to infection alters neurodevelopment, potentially increasing susceptibility to schizophrenia.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V et al (2004). Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry 161: 889–895. [DOI] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD (2008). Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry 63: 1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhimschi IA, Buhimschi CS, Weiner CP (2003). Protective effect of N-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. Am J Obstet Gynecol 1881: 203–208. [DOI] [PubMed] [Google Scholar]
- Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Wagner RL, Yolken RH (2001). Maternal Cytokine Levels during Pregnancy and Adult Psychosis. Brain Behav Immun 15: 411–420. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivy JR, Crowe RJ et al (2012). Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alpha administration. Arch Gen Psychiatry 69: 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrizo E, Fernández V, Quintero J, Connell L, Rodríguez Z, Mosquera M (2008). Coagulation and inflammation markers during atypical or typical antipsychotic treatment in schizophrenia patients and drug-free first-degree relatives. Schizophr Res 103: 83–93. [DOI] [PubMed] [Google Scholar]
- Chaudhry IB, Husain N, ur Rahman R, Husain MO, Hamirani MM, Kazmi A et al (2015). A randomised double-blind placebo-controlled 12- week feasibility trial of methotrexate added to treatment as usual in early schizophrenia: study protocol for a randomised controlled trial. Trials 16: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AT, Chibnall JT, Nasrallah HA (2015). A meta-analysis of placebo-controlled trials of omega-3 fatty acid augmentation in schizophrenia: possible stage-specific effects. Ann Clin Psychiatry 27: 289–296. [PubMed] [Google Scholar]
- Chen SJ, Chao YL, Chen CY, Chang CM, Wu EC, Wu CS et al (2012). Prevalence of autoimmune diseases in in-patients with schizophrenia: nationwide population-based study. Br J Psychiatry 200: 374–380. [DOI] [PubMed] [Google Scholar]
- Chen, da C, Qi LY, Xiu MH, Cao LY, Wang F et al (2008). Elevated serum levels of tumor necrosis factor-alpha in clozapine-associated obesity in chronic schizophrenia. Schizophr Res 106: 367–368. [DOI] [PubMed] [Google Scholar]
- Chiappelli J, Pocivavsek A, Nugent KL, Notarangelo FM, Kochunov P, Rowland LM et al (2014). Stress-induced increase in kynurenic acid as a potential biomarker for patients with schizophrenia and distress intolerance. JAMA Psychiatry 71: 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching ASC, Kuhnast B, Damont A, Roeda D, Tavitian B, Dollé F (2012). Current paradigm of the 18-kDa translocator protrain (TSPO) as a molecular target for PET imaging in neuroinflammation and neurodegenerative diseases. Insights Imaging 3: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GB, Kim YS, Wong H, Kim S, Kim H, Kim SV et al (2016). The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351: 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MC, Tanskanen A, Huttunen M, Whittaker JC, Cannon M (2009). Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry 166: 1025–1030. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD et al (2015). Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry 173: 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza-Nashat M, Zhao ML, Suh HS, Morgan K, Natividad R, Morgello S et al (2009). Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathology and Applied Neurobiology 35: 306–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Ishizuka K, Kano SI, Edwards JA, Seifuddin FT, Shimano MA et al (2013). Marked reduction of soluble superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with recent-onset schizophrenia. Mol Psychiatry 18: 10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Ambinder EB, Ward RE, Minn I, Vranesic M et al (2016). In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [11C]DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry 6: e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle P, Tran N, Fung JN, Summers BL, Rofe AM (2009). Maternal dietary zinc supplementation prevents aberrant behavior in an object recognition task in mice offspring exposed to LPS in early pregnancy. Behav Brain Res 197: 210–218. [DOI] [PubMed] [Google Scholar]
- Demontis D, Nyegaard M, Buttenschøn HN, Hedemand A, Pedersen CB, Grove J et al (2011). Association of GRIN1 and GRIN2A-D with schizophrenia and genetic interaction with maternal herpes simplex virus-2 infection affecting disease risk. Am J Med Genet B Neuropsychiatr Genet 156B: 913–922. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Schroeder J, Khushalani S, Yolken R (2014). Mortality in schizophrenia: clinical and serological predictors. Schizophr Bull 40: 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov DH (2011). Correlation or coincidence between monocytosis and worsening of psychosis symptoms in veterans with schizophrenia? Schizophr Res 126: 306–307. [DOI] [PubMed] [Google Scholar]
- Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M (2009). Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol 19: 220–230. [DOI] [PubMed] [Google Scholar]
- Doorduin J, de Vries EFJ, Willemsen ATM, de Groot JC, Dierckx RA, Klein HC (2009). Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med 50: 1801–1807. [DOI] [PubMed] [Google Scholar]
- Drexhage RC, van der Heul-Nieuwenhuijsen L, Padmos RC, van Beveren N, Cohen D, Versnel MA et al (2010). Inflammatory gene expression in monocytes of patients with schizophrenia: overlap and difference with bipolar disorder. A study in naturalistically treated patients. Int J Neuropsychopharmacol 13: 1369–1381. [DOI] [PubMed] [Google Scholar]
- Eaton W, Byrne M, Ewald H, Mors O, Chen CY, Agerbo E et al (2006). Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry 163: 521–528. [DOI] [PubMed] [Google Scholar]
- Eaton W, Pederson M, Nielsen P, Mortensen P (2010). Autoimmune diseases, bipolar disorder, and non-affective psychosis. Bipolar Disord 12: 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR (2010). Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry 68: 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G (2001). Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett 313: 96–98. [DOI] [PubMed] [Google Scholar]
- Ezeoke A, Mellor A, Buckley P, Miller BJ (2013). A systematic quantitative review of blood autoantibody elevations in schizophrenia. Schizophrenia Res 150: 245–251. [DOI] [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM et al (2007). Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol Psychiatry 62: 1324–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X et al (2015). Inflammation is associated with decreased functional connectivity within corticostriatal reward citcuitry in depression. Mol Psychiatry 21: 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellerhoff B, Laumbacher B, Mueller N, Gu S, Wank R (2007). Associations between Chlamydophila infections, schizophrenia and risk of HLA-A10. Mol Psychiatry 12: 264–272. [DOI] [PubMed] [Google Scholar]
- Fernandes BS, Steiner J, Bernstein HG, Dodd S, Pasco JA, Dean OM et al (2016). C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry21: 554–564.. [DOI] [PubMed]
- Flatow J, Buckley P, Miller B (2013). Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry 74: 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simoes MJ, Cerri PS (2015). Biology of bone tissue: structure, function, and factors that influence bone cells. BioMed Res Int 2015: 421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O'Connor J, Lawson M, Micon C, Lestage J et al (2007). Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology 32: 516–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Joshi D, Fillman SG, Weickert CS (2014). High white matter neuron density with elevated cortical cytokine expression in schizophrenia. Biol Psychiatry 75: e5–e7. [DOI] [PubMed] [Google Scholar]
- Garay PA, Hsiao EY, Patterson PH, McAllister AK (2013). Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Beahv Immun 31: 54–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Li Z, Chang S, Wang J (2014). Association of interleukin-10 polymorphisms with schizophrenia: a meta-analysis. PLoS One9: e90407.. [DOI] [PMC free article] [PubMed]
- Gardner RM, Dalman C, Wicks S, Lee BK, Karlsson H (2013). Neonatal levels of acute phase proteins and later risk of non-affective psychosis. Transl Psychiatry19: e228.. [DOI] [PMC free article] [PubMed]
- Gaskell EA, Smith JE, Pinney JW, Westhead DR, McConkey GA (2009). A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS One 4: e4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar PA, Bustamante ML, Silva H, Aboitiz F (2009). Molecular mechanisms underlying glutamatergic dysfunction in schizophrenia: therapeutic implications. J Neurochem 111: 891–900. [DOI] [PubMed] [Google Scholar]
- Gaughran F, O'Neill E, Sham P, Daly RJ, Shanahan F (2002). Soluble interleukin 2 receptor levels in families of people with schizophrenia. Schizophr Res 56: 235–239. [DOI] [PubMed] [Google Scholar]
- Gilvarry C, Sham P, Jones P, Cannon M, Wright P, Lewis SW et al (1996). Family history of autoimmune diseases in psychosis. Schizophr Res 19: 33–40. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S et al (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330: 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R et al (2013). Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 339: 1095–1099. [DOI] [PubMed] [Google Scholar]
- Giovanoli S, Weber-Stadbauer U, Scvhedlowski M, Meyer U, Engler H (2016). Prenatal immune activation causes hippocampal synaptic deficits in the absence of overt microglia anomalies. Brain Beahv Immun 55: 25–38. [DOI] [PubMed] [Google Scholar]
- Girard S, Tremblay L, Lepage M, Sebire G (2010). IL-1 receptor antagonist protects against placental and neurodevelopmental defects induced by maternal inflammation. J Immunol 184: 3997–4005. [DOI] [PubMed] [Google Scholar]
- Goldsmith D, Rapaport MH, Miller BJ (2016). Meta-analysis of cytokine alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Mol Psychiatry In press. Meta-analysis of 68 studies which found that levels of two cytokines (IL-6 and TNF-α), one soluble cytokine receptor (sIL-2R), and one cytokine receptor antagonist (IL-1RA) were significantly increased in acutely ill patients with schizophrenia, bipolar mania and MDD compared with controls.. [DOI] [PMC free article] [PubMed]
- Graham KL, Bodenheimer CM, Ezeoke A, Buckley PF, Miller BJ (2014). Urinary tract infections in acute relapse of psychosis. JClin Psychiatry 75: 379–385. [DOI] [PubMed] [Google Scholar]
- Grüber L, Bunse T, Weidinger E, Reichard H, Müller N (2014). Adjunctive recombinant human interferon gamma-1b for treatment-resistant schizophrenia in 2 patients. J Clin Psychiatry 75: 266–1267. [DOI] [PubMed] [Google Scholar]
- Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P et al (2007). Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci USA 104: 16621–16626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack M, Hinze-Selch D, Fenzel T, Kraus T, Kuhn M, Schuld A et al (1999). Plasma levels of cytokines and soluble cytokine receptors in psychiatric patients upon hospital admission: Effects of confounding factors and diagnosis. J Psychiatr Res 33: 407–418. [DOI] [PubMed] [Google Scholar]
- Haroon E, Felger JC, Woolwine BJ, Chen X, Parekh S, Spivey JR et al (2015). Age-related increases in basal ganglia glutamate are associated with TNF, reduced motivation and decreased psychomotor speed during IFN-alpha treatment: preliminary findings. Brain Behav Immun 46: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Voon V, Cercignani M, Cooper EA, Pessiglione M, Critchley HD (2015). A neurocomputational account of how inflammation enhances sensitivity to punishments versus rewards. Biol Psychiatry 80: 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey L, Boksa P (2014). Additive effects of maternal iron deficiency and prenatal immune activation on adult behaviors in rat offspring. Brain Behavior Immun 40: 27–37. [DOI] [PubMed] [Google Scholar]
- Havlicek J, Gasova ZG, Smith AP, Zvara K, Flegr J (2001). Decrease of psychomotor performance in subjects with latent ‘asymptomatic' toxoplasmosis. Paristology 122: 515–520. [DOI] [PubMed] [Google Scholar]
- Hayes LN, Severance EG, Leek JT, Gressitt KL, Rohleder C, Coughlin JM et al (2014). Inflammatory molecular signature associated with infectious agents in psychosis. Schizoprhenia Bulletin 40: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoseth EZ, Westlye LT, Hope S, Dieset I, Aukrust P, Melle I et al (2016). Association between cytokine levels, verbal memory and hippocampus volume in psychotic disorders and healthy controls. Acta Psychiatr Scand 133: 53–62. [DOI] [PubMed] [Google Scholar]
- Hudson ZD, Miller BJ (2016). Meta-Analysis of cytokine and chemokine genes in schizophrenia. submitted to clinical schizophrenia & related psychoses. (In press). [DOI] [PubMed]
- Hutchison WM, Aitken PP, Wells BW (1980). Chronic Toxoplasma infections and motor performance in the mouse. Ann Trop Med Parasitol 74: 507–510. [DOI] [PubMed] [Google Scholar]
- Ingman WV, Robertson SA (2009). The essential roles of TGFB1 in reproduction. Cytokine Growth Factor Rev 20: 233–239. [DOI] [PubMed] [Google Scholar]
- Jiang L, Lindpaintner K, Li H-F, Gu N-F, Langen H, He L et al (2003). Proteomic analysis of the cerebrospinal fluid of patients with schizophrenia. Amino Acids 25: 49–57. [DOI] [PubMed] [Google Scholar]
- Juckel G, Friedel E, Koslowski M, Witthaus H, Ozgurdal S, Gudlowski Y et al (2012). Ventral striatal activation during reward processing in subjects with ultra-high risk for schizophrenia. Neuropsychobiology 66: 50–56. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Filonov D, Wustenberg T, Villringer A et al (2006). Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, but not atypical, neuroleptics. Psychopharmacology 187: 222–228. [DOI] [PubMed] [Google Scholar]
- Kalmady SV, Venkatasubramanian G, Shivakumar V, Gautham S, Subramaniam A, Jose DA et al (2014). Relationship between Interleukin-6 gene polymorphism and hippocampal volume in antipsychotic-naïve schizophrenia: evidence for differential susceptibility? PLoS One 9: e96021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano S, Nwulia E, Niwa M, Chen Y, Sawa A, Cascella N (2011). Altered MHC class I expression in dorsolateral prefrontal cortex of nonsmoker patients with schizophrenia. Neurosci Res 71: 289–293. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Woods SW, Petkova E, Cornblatt B, Corcoran CM, Chen H et al (2015). D-serine for the treatment of negative symptoms in individuals at clinical high risk of schizophrenia: a pilot, double-blind, placebo-controlled, randomized parallel group mechanistic proof-of-concept trial. Lancet Psychiatry 2: 403–412. [DOI] [PubMed] [Google Scholar]
- Kegel ME, Bhat M, Skogh E, Samuelsson M, Lundberg K, Dahl ML et al (2014). Imbalanced kynurenine pathway in schizophrenia. Int J Tryptophan Res 7: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenk M, Selvanathan T, Rao N, Suridjan I, Rusjan P, Remingston G et al (2015). Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo study with [18 F]-FEPPA. Schizophr Bull 41: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A (2011). Physiology of microglia. Physiol Rev 91: 461–553. [DOI] [PubMed] [Google Scholar]
- Khandaker** GM, Pearson RM, Zammit S, Lewis G, Jones PB (2014). Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry 71: 1121–1128 Cohort study of about 4500 individuals that found blood IL-6 levels at age 9 were associated with risk of psychotic experiences and fulminant psychotic disorder at age 18 in a dose-dependent fashion.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Miller BJ (2013). Inflammation and schizophrenia. Schizophr Bull 39: 1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluge M, Schuld A, Schacht A, Himmerich H, Dalal MA, Wehmeier PM et al (2009). Effects of clozapine and olanzapine on cytokine systems are closely linked to weight gain and drug-induced fever. Psychoneuroendocrinology 34: 118–128. [DOI] [PubMed] [Google Scholar]
- Koola MM (2016). Methodological issues in cytokine measurement in schizophrenia. Indian J Psychol Med 38: 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski J, Blada P, Kucia K, Madej A, Herman ZS (2001). Neuroleptics normalize increased release of interleukin-1 beta and tumor necrosis factor-alpha from monocytes in schizophrenia. Schizophr Res. 50: 169–175. [DOI] [PubMed] [Google Scholar]
- Krause D, Matz J, Weidinger E, Wagner J, Wildenauer A, Obermeier M et al (2010). The association of infectious agents and schizophrenia. World J Biol Psychiatry 11: 739–743. [DOI] [PubMed] [Google Scholar]
- Krause DL, Wagner JK, Wildenauer A, Matz J, Weidinger E, Riedel M et al (2012. a). Intracellular monocytic cytokine levels in schizophrenia show an alteration of IL-6. Eur Arch Psychiatry Clin Neurosci 262: 393–401. [DOI] [PubMed] [Google Scholar]
- Krause D, Wagner J, Matz J, Weidinger E, Obermeier M, Riedel M et al (2012. b). Monocytic HLA DR antigens in schizophrenic patients. Neurosci Res 72: 87–93. [DOI] [PubMed] [Google Scholar]
- Kucharska-Mazur J, Tarnowski M, Dołęgowska B, Budkowska M, Pędziwiatr D, Jabłoński M et al (2014). Novel evidence for enhanced stem cell trafficking in antipsychotic-naïve subjects during their first psychotic episode. J Psychiatr Res 49: 18–24. [DOI] [PubMed] [Google Scholar]
- Laan W, Grobbee DE, Selten JP, Heijnen CJ, Kahn RS, Burger H (2010). Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 71: 520–527. [DOI] [PubMed] [Google Scholar]
- Labad J, Stojanovic-Pérez A, Montalvo I, Solé M, Cabezas Á, Ortega L et al (2015). Stress biomarkers as predictors of transition to psychosis in at-risk mental states: roles for cortisol, prolactin and albumin. J Psychiatr Res 60: 163–169. [DOI] [PubMed] [Google Scholar]
- Labouesse MA, Langhans W, Meyer U (2015). Long-term pathological consequences of prenatal infection: beyond brain disorders. Am J Physiol Regul Integr Comp Physiol 309: R1–R12. [DOI] [PubMed] [Google Scholar]
- Labouesse MA, Dong E, Grayson DR, Guidotti A, Meyer U (2015). Maternal immune activation induces GAD1 and GAD2 promoter remodeling in the offspring prefrontal cortex. Epigenetics 10: 1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachance LR, McKenzie K (2014). Biomarkers of gluten sensitivity in patients with non-affective psychosis: a meta-analysis. Schizophr Res 152: 521–527. [DOI] [PubMed] [Google Scholar]
- Laney D, Philip N, Miller BJ (2015). Recurrent urinary tract infections in acute psychosis. Schizophr Res 164: 275–276. [DOI] [PubMed] [Google Scholar]
- Lanté F, Meunier J, Guiramand J, De Jesus Ferreira MC, Cambonie G, Aimar R et al (2008). Late N-acetylcysteine treatment prevents the deficits induced in the offspring of dams exposed to an immune stress during gestation. Hippocampus 18: 602–609. [DOI] [PubMed] [Google Scholar]
- Laskaris LE, Di Biase MA, Everall I, Chana G, Christopoulos A, Skafidas E et al (2016). Microglial activation and progressive brain changes in schizophrenia. Br J Pharmacol 173: 666–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Peng H, Gelbart T, Wang L, Beutler E (2010). Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci 102: 1906–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenczowski MJ, Bluthe RM, Roth J, Rees GS, Rushforth DA, van Dam AM et al (1999). Central administration of rat IL-6 induces HPA activation and fever but not sickness behavioral in rats. Am J Physiol 276: R652–R658. [DOI] [PubMed] [Google Scholar]
- Levine J, Gutman J, Feraro R, Levy P, Kimhi R, Leykin I et al (1997). Side effect profile of azathioprine in the treatment of chronic schizophrenic patients. Neuropsychobiology 36: 172–176. [DOI] [PubMed] [Google Scholar]
- Liu L, Jia F, Yuan G, Chen Z, Yao J, Li H et al (2010). Tyrosine hydroxylase, interleukin-1beta and tumor necrosis factor-alpha are overexpressed in peripheral blood mononuclear cells from schizophrenia patients as determined by semi-quantitative analysis. Psychiatry Res 176: 1–7. [DOI] [PubMed] [Google Scholar]
- Löffler S, Löffler-Ensgraber M, Fehsel K, Klimke A (2010. a). Clozapine therapy raises serum concentrations of high sensitive C-reactive protein in schizophrenic patients. Int Clin Psychopharmacol 25: 101–106. [DOI] [PubMed] [Google Scholar]
- Löffler S, Klimke A, Kronenwett R, Kobbe G, Haas R, Fehsel K (2010. b). Clozapine mobilizes CD34+ hematopoietic stem and progenitor cells and increases plasma concentration of interleukin 6 in patients with schizophrenia. J Clin Psychopharmacol 30: 591–595. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, Kenis G, De Jong R, Smith RS, Meltzer HY (1997). In vivo immunomodulatory effects of clozapine in schizophrenia. Schizophr Res 26: 221–225. [DOI] [PubMed] [Google Scholar]
- Magalhães PV, Dean O, Andreazza AC, Berk M, Kapczinski F (2016). Antioxidant treatments for schizophrenia. Cochrane Database Syst Rev 2: CD008919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH (2008). IFN-alpha induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun 22: 870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manu P, Correll CU, Wampers M, Mitchell AJ, Probst M, Vancampfort D et al (2014). Markers of inflammation in schizophrenia: association vs. causation. World Psychiatry 13: 189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K (2013). Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: the importance of microglia phenotype. Neurobiol Dis 54: 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LF, Kem WR, Freedman R (2004). Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl) 174: 54–64. [DOI] [PubMed] [Google Scholar]
- Martínez-Gras I, García-Sánchez F, Guaza C, Rodríguez-Jiménez R, Andrés-Esteban E, Palomo T et al (2012). Altered immune function in unaffected first-degree biological relatives of schizophrenia patients. Psychiatry Res 200: 1022–1025. [DOI] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Parolaro D (2006). Cannabinoids, immune system and cytokine network. Curr Pharm Des 12: 3135–3146. [DOI] [PubMed] [Google Scholar]
- Matcovitch-Natan O, Winter DR, Giladi A, Aguilar SV, Spinrad A, Sarrazin S et al (2016). Microglia development follows a stepwise program to regulate brain homeostasis. Science 353: aad8670. [DOI] [PubMed] [Google Scholar]
- Mayilyan KR, Weinberger DR, Sim RB (2008). The complement system in schizophrenia. Drug News Perspect 21: 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams C, Leonard BE (1993). Neutrophil and monocyte phagocytosis in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 17: 971–984. [DOI] [PubMed] [Google Scholar]
- Meyer U (2013). Developmental neuroinflammation and schizophrenia. Progress in Neuro-Psychopharmacology &. Biological Psychiatry 42: 20–34. [DOI] [PubMed] [Google Scholar]
- Meyer U (2014). Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry 75: 307–315 Comprehensive review of developmental immune activation models in rodents.. [DOI] [PubMed] [Google Scholar]
- Miller B, Buckley P, Seabolt W, Mellor A, Kirkpatrick B (2011). Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biological Psychiatry 70: 663–671 Meta-analysis of 40 studies which found that blood cytokine alterations were similar in magnitude and direction for patients with first-episode psychosis and acutely ill patients with schizophrenia compared to controls, and that cytokine alterations vary based on clinical status.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Graham KL, Bodenheimer CM, Culpepper NH, Waller JL, Buckley PF (2013). A prevalence study of urinary tract infections in acute relapse of schizophrenia. J Clin Psychiatry 73: 271–277. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Bodenheimer CM, Culpepper NH, Graham KL, Buckley PF (2013). Differential white blood cell counts may predict urinary tract infection in acute non-affective psychosis. Schizophr Res 147: 400–401. [DOI] [PubMed] [Google Scholar]
- Miller B, Culpepper N, Rapaport M, Buckley P (2013). Prenatal inflammation and neurodevelopment in schizophrenia: a review of human studies. Prog Neuro-Psychopharmacol Biol Psychiatry 42: 92–100. [DOI] [PubMed] [Google Scholar]
- Miller B, Gassama B, Sebastian D, Buckley P, Mellor A (2013). Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 73: 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Mellor A, Buckley P (2013). Total and differential white blood cell counts, high-sensitivity c-reactive protein, and the metabolic syndrome in non-affective psychoses. Brain Behavior Immun 31: 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Culpepper N, Rapaport MH (2014). C-Reactive Protein in Schizophrenia: A Review and Meta-Analysis. Clin Schizophr Relat Psychoses 7: 223–230. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Timonen M, Isohanni M (2014). Cytokine abnormalities, inflammation and psychosis in the northern finland 1966 birth cohort. Eur Psychiatry 29: S519. [Google Scholar]
- Miller** BJ, Dias JK, Lemos HP, Buckley PF (2016). An Open-Label, Pilot Trial of Adjunctive Tocilizumab in Schizophrenia. J Clin Psychiatry 77: 275–276 Open-label trial of adjunctive anti-IL-6 receptor monoclonal antibody immunotherapy was associated with improved cognition in schizophrenia.. [DOI] [PubMed] [Google Scholar]
- Mondelli V, Cattaneo A, Belvederi Murri M, Di Forti M, Handley R, Hepgul N et al (2011). Stress and inflammation reduce brain-derived neurotrophic factor expression in first-episode psychosis: a pathway to smaller hippocampal volume. J Clin Psychiatry 72: 1677–1684 In patients with first-episode psychosis, lower BDNF expression, increased IL-6 expression, and increased cortisol levels all significantly and independently predicted a smaller left hippocampal volume.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monji A, Kato T, Kanba S (2009). Cytokines and schizophrenia: microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci 63: 257–265. [DOI] [PubMed] [Google Scholar]
- Monroe JM, Buckley PF, Miller BJ (2015). Meta-analysis of anti-toxoplasma gondii igm antibodies in acute psychosis. Schizophr Bull 41: 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone P, Fabrazzo M, Tortorella A, Maj M (1997). Plasma levels of inteleukin-6 and tumor necrosis factor alpha in chronic schizophrenia: effects of clozapine treatment. Psychiatry Res 71: 11–17. [DOI] [PubMed] [Google Scholar]
- Muller N, Ulmschneider M, Scheppach C, Schwarz MJ, Ackenheil M, Möller HJ et al (2004). COX-2 inhibition as a treatment approach in schizophrenia: immunological considerations and clinical effects of celecoxib add-on therapy. Eur Arch Psychiatry Clin Neurosci 254: 14–22. [DOI] [PubMed] [Google Scholar]
- Müller N, Wagner JK, Krause D, Weidinger E, Wildenauer A, Obermeier M et al (2012). Impaired monocyte activation in schizophrenia. Psychiatry Res 198: 341–346. [DOI] [PubMed] [Google Scholar]
- Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O (2013). Neuroinflammation and psychiatric illness. J Neuroinflammation 10: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar** S, Pearlman DM (2015). Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res 161: 102–112 Systematic review of fifteen studies found consistent evidence in neuroimaging and neuropathological studies of an association between schizophrenia and microglial activation, particularly in white rather than gray matter regions.. [DOI] [PubMed] [Google Scholar]
- Narendran R, Frankle WG (2016). Comment on Analyses and Conclusions of “Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [11C]PBR28 PET brain imaging study”. Am J Psychiatry 173: 536–537. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK et al (2004). IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113: 1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MO, Rostrup E, Wulff S, Bak N, Lublin H, Kapur S et al (2012). Alterations of the brain reward system in antipsychotic naïve schizophrenia patients. Biol Psychiatry 71: 898–905. [DOI] [PubMed] [Google Scholar]
- Nielsen PR, Meyer U, Mortensen PB (2016). Individual and combined effects of maternal anemia and prenatal infection on risk for schizophrenia in offspring. Scz Res 172: 35–40. [DOI] [PubMed] [Google Scholar]
- Nielsen PR, Agerbo E, Skogstrand K, Hougaard DM, Meyer U, Mortensen PB (2015). Neonatal levels of inflammatory markers and later risk of schizophrenia. Biol Psychiatry 77: 548–555. [DOI] [PubMed] [Google Scholar]
- Nielsen PR, Benros ME, Mortensen PB (2014). Hospital contacts with infection and risk of schizophrenia: a population-based cohort study with linkage of Danish national registers. Schizophr Bull 40: 1526–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkilä HV, Müller K, Ahokas A, Miettinen K, Rimón R, Andersson LC (1999). Accumulation of macrophages in the CSF of schizophrenic patients. Am J Psychiatry 156: 1725–1729. [DOI] [PubMed] [Google Scholar]
- Nikkilä HV, Müller K, Ahokas A, Rimón R, Andersson LC (2001). Increased frequency of activated lymphocytes in the cerebrospinal fluid of patients with acute schizophrenia. Schizophr Res 49: 99–105. [DOI] [PubMed] [Google Scholar]
- Nilsson LK, Linderholm KR, Engberg G, Paulson L, Blennow K, Lindström LH et al (2005). Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res 80: 315–322. [DOI] [PubMed] [Google Scholar]
- Nitta M, Kishimoto T, Müller N, Weiser M, Davidson M, Kane JM et al (2013). Adjunctive use of nonsteroidal anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Schizophr Bull 39: 1230–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308: 1314–1318. [DOI] [PubMed] [Google Scholar]
- Nunes SO, Matsuo T, Kaminami MS, Watanabe MA, Reiche EM, Itano EN (2006). An autoimmune or an inflammatory process in patients with schizophrenia, schizoaffective disorder, and in their biological relatives. Schizophr Res 84: 180–182. [DOI] [PubMed] [Google Scholar]
- O'Connell KE, Thakore J, Dev KK (2014). Pro-inflammatory cytokine levels are raised in female schizophrenia patients treated with clozapine. Schizophr Res 156: 1–8. [DOI] [PubMed] [Google Scholar]
- O'Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME et al (2009). To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun 23: 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintlia MK, Paintlia AS, Contreras MA, Singh I, Singh AK (2008). Lipopolysaccharide-induced peroxisomal dysfunction exacerbates cerebral white matter injury: attenuation by N-acetylcysteine. Exp Neurol 210: 560–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Kwon YJ, Jeong HY, Lee HY, Hwangbo Y, Yoon HJ et al (2012). Association between Intracellular Infectious Agents and Schizophrenia. Clin Psychopharmacol Neurosci 10: 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce BD, Hubbard S, Rivera HN, Wilkins PP, Fisch MC, Hopkins MH et al (2013). Toxoplasma gondii exposure affects neural processing speed as measured by acoustic startle latency in schizophrenia and controls. Scz Res 150: 258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman DM, Najjar S (2014). Meta-analysis of the association between N-methyl-d-aspartate receptor antibodies and schizophrenia, schizoaffective disorder, bipolar disorder, and major depressive disorder. Schizophr Res 157: 249–258. [DOI] [PubMed] [Google Scholar]
- Perkins** DO, Jeffries CD, Addington J, Bearden CE, Cadenhead KS, Cannon TD et al (2015). Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: preliminary results from the NAPLS project. Schizophr Bull 41: 419–428 A multiplex panel of blood inflammation, oxidative stress, and hypothalamic-pituitary axis markers was a strong predictor of progression to psychosis in clinical high risk subjects.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pershing ML, Bortz DM, Pocivavsek A, Fredericks PJ, Jorgensen CV, Vunck SA et al (2015). Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: Implications for schizophrenia. Neuropharmacology 90: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletnikov MV, Rubin SA, Schwartz GJ, Carbone KM, Moran TH (2000). Effects of neonatal rat Borna disease virus (BDV) infection on the postnatal development of the brain monoaminergic systems. Brain Res Dev Brain Res 119: 179–185. [DOI] [PubMed] [Google Scholar]
- Pocivavsek A, Wu HQ, Elmer GI, Bruno JP, Schwarcz R (2012). Pre- and post-natal exposure to kynurenine causes cognitive deficits in adulthood. Eur J Neurosci 35: 1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollmächer T, Hinze-Selch D, Mullington J (1996). Effects of clozapine on plasma cytokine and soluble cytokine receptor levels. J Clin Psychopharmacol 6: 403–409. [DOI] [PubMed] [Google Scholar]
- Qin H, Zhang L, Xu G, Pan X (2013). Lack of association between TNF rs1800629 polymorphisms and schizophrenia risk: a meta-analysis. Psychiatry Research209: 314–319.. [DOI] [PubMed]
- Raison CL, Rye DB, Woolwine BJ, Vogt GJ, Bautista BM, Spivey JR et al (2010). Chronic interferon-alpha administration disrupts sleep continuity and depth in patients with hepatitis C: association with fatigue, motor slowing, and increased evening cortisol. Biol Psychiatry 68: 942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran A, Venkatasubramanian G, Berk M, Debnath M (2015). Mitochondrial dysfunction in schizophrenia: pathways, mechanisms and implications. Neurosci Biobehav Rev 48: 10–21. [DOI] [PubMed] [Google Scholar]
- Ravikumar A, Deepadevi KV, Arun P, Manojkumar V, Kurup PA (2000). Tryptophan and tyrosine catabolic pattern in neuropsychiatric disorders. Neurol India 48: 231–238. [PubMed] [Google Scholar]
- Réus G, Fries G, Stertz L, Badawy M, Passos I, Barichello T et al (2015). The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 300: 141–154. [DOI] [PubMed] [Google Scholar]
- Røge R, Møller BK, Andersen CR, Correll CU, Nielsen J (2012). Immunomodulatory effects of clozapine and their clinical implications: what have we learned so far. Schizophr Res 140: 204–213. [DOI] [PubMed] [Google Scholar]
- Rwegellera GG, Fernando KA, Okong'o O (1982). Bactericidal activity of neutrophils of schizophrenic patients. Med J Zambia 16: 21–22. [PubMed] [Google Scholar]
- Saha S, Chant D, McGrath J (2007). A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry 64: 1123–1131. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S (1994). Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res 65: 221–229. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M (2012). The mysterious motivational functions of mesolimbic dopamine. Neuron 76: 470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos Sória Ld, Moura Gubert Cd, Ceresér KM, Gama CS, Kapczinski F (2012). Increased serum levels of C3 and C4 in patients with schizophrenia compared to eutymic patients with bipolar disorder and healthy. Rev Bras Psiquiatr 34: 119–120. [PubMed] [Google Scholar]
- Sathyasaikumar KV, Stachowski EK, Wonodi I, Roberts RC, Rassoulpour A, McMahon RP et al (2011). Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull. 37: 1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature 511: 421–427SI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Bertsch T, Tost H, Bergmann A, Henning U, Klimke A et al (2005). Increased serum interleukin-1beta and interleukin-6 in elderly, chronic schizophrenic patients on stable antipsychotic medication. Neuropsychiatr Dis Treat 1: 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Hasan A, Gruber O, Falkai P (2011). Schizophrenia as a disorder of disconnectivity. Eur. Arch. Psychiatry Clin. Neurosci 261: S150–S154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC (2001). Increased cortical kynurenate content in schizophrenia. Biol Psychiatry 50: 521–530. [DOI] [PubMed] [Google Scholar]
- Schwieler L, Larsson MK, Skogh E, Kegel ME, Orhan F, Abdelmoaty S et al (2015). Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia – significance for activation of the kynurenine pathway. J Psychiatry Neurosci. 2: 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K et al (2016). Schizophrenia risk from complex variation of complement component 4. Nature 530: 177–183 Series of experiments that linked together how a specific variant of the C4 gene, which encodes the complement protein C4, may increase schizophrenia risk by influencing synaptic pruning during critical periods of brain development.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Halling M, Stallings CR, Origoni AE, Vaughan C et al (2012). Complement C1q formation of immune complexes with milk caseins and wheat glutens in schizophrenia. Neurobiol Dis 48: 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I et al (2009). Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 460: 753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIRS Symposium. Potential role of NMDA-receptor antibodies in schizophrenia: overlap and distinction from NMDA receptor encephalitis. Symposium presentation, 4th Schizophrenia International Research Society Conference; Florence, Italy. April 8, 2014..
- Shibuya M, Watanabe Y, Nunokawa A, Egawa J, Kaneko N, Igeta H et al (2014). Interleukin 1 beta gene and risk of schizophrenia: detailed case-control and family-based studies and an updated meta-analysis. Hum Psychopharmacol 29: 31–37. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH (2007). Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 27: 10695–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solbrig MV, Koob GF, Fallon JH, Reid S, Lipkin WI (1996). Prefrontal cortex dysfunction in Borna disease virus (BDV)-infected rats. Biol Psychiatry 40: 629–636. [DOI] [PubMed] [Google Scholar]
- Sommer** IE, van Westrhenen R, Begemann MJ, de Witte LD, Leucht S, Kahn RS (2014). Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull 40: 181–191 Meta-analysis of 26 randomized controlled trials of anti-inflammatory agents found evidence for significant effects with aspirin, estrogens, and N-acetyl cysteine.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth S, Ravi V, Poornima KS, Shetty KT, Gangadhar BN, Janakiramaiah N (1994). Viral antibodies in recent onset, nonorganic psychoses: correspondence with symptomatic severity. Biol Psychiatry 36: 517–521. [DOI] [PubMed] [Google Scholar]
- Steiner J, Mawrin C, Ziegeler A, Bielau H, Ullrich O, Bernstein HG et al (2006). Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol 112: 305–316. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C et al (2008). Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res 42: 151–157. [DOI] [PubMed] [Google Scholar]
- Stojanovic A, Martorell L, Montalvo I, Ortega L, Monseny R, Vilella E et al (2014). Increased serum interleukin-6 levels in early stages of psychosis: associations with at-risk mental states and the severity of psychotic symptoms. Psychneuroendocrinology 41: 23–32. [DOI] [PubMed] [Google Scholar]
- Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R et al (2010). Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. Int J Neuropsychopharmacol 13: 943–950. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Bartko JJ, Lun ZR, Yolken RH (2007). Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull 33: 729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upthegrove R, Manzanares-Teson N, Barnes NM (2014). Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res 155: 101–108. [DOI] [PubMed] [Google Scholar]
- Vallee BL, Falchuk KH (1993). The biochemical basis of zinc pathophysiology. Physiol Rev 73: 79–118. [DOI] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E et al (2008). Microglia activation in recent-onset schizophrenia: a quantitative (R-[11C]PK11195 Positron Emission Tomography Study. Biol Psychiatry 64: 820–822. [DOI] [PubMed] [Google Scholar]
- Vichaya EG, Hunt SC, Danzer R (2014). Lipopolysaccharide reduces incentive motivation while boosting preference for high reward in mice. Neuropsychopharmacology 39: 2884–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk** DW, Chitrapu A, Edelson JR, Roman KM, Moroco AE, Lewis DA (2015). Molecular mechanisms and timing of cortical immune activation in schizophrenia. Am J Psychiatry 172: 1112–1121 Study found evidence of increased mRNA levels of immune-related cytokines and transcription factors in the prefrontal cortex may be attributable to adult, but not prenatal, immune activation.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe GE, Ward H, Mwamburi M, Dinh D, Bhalchandra S, Wanke C et al (2014). Associations of cocaine use and HIV infection with the intestinal microbiota, microbial translocation, and inflammation. J Stud Alcohol Drugs 75: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Stadlbauer U, Richetto J, Labouesse MA, Bohacek J, Mansuy IM, Meyer U (2016). Transgenerational transmission and modification of pathological traits induced by prenatal immune activation. Molecular Psychiatry advanced online publication. [DOI] [PubMed]
- Wang AK, Miller BJ (2016). Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. In preparation. [DOI] [PMC free article] [PubMed]
- Webster JP, McConkey GA (2010). Toxoplasma gondii-altered host behavior: clues as to mechanism of action. Folia Parisitol 57: 95–104. [DOI] [PubMed] [Google Scholar]
- Witting PA (1979). Learning capacity and memory of normal and Toxoplasma-infected laboratory rats and mice. Z Parasitenkd 61: 29–51. [DOI] [PubMed] [Google Scholar]
- Wonodi I, Stine OC, Sathyasaikumar KV, Roberts RC, Mitchell BD, Hong LE et al (2011). Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch Gen Psychiatry 68: 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonodi I, McMahon RP, Krishna N, Mitchell BD, Liu J, Glassman M et al (2014). Influence of kynurenine 3-monooxygenase (KMO) gene polymorphism on cognitive function in schizophrenia. Schizophr Res 160: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, He L (2010). Convergent evidence shows a positive association of interleukin-1 gene complex locus with susceptibility to schizophrenia in the caucasian population. Schizophr Res 120: 131–142. [DOI] [PubMed] [Google Scholar]
- Yang L, Li FF, Han YC, Jia B, Ding Y (2015). Cannabinoid receptor CB2 is involved in tetrahydrocannabinol-induced anti-inflammation against lipopolysaccharide in MG-63 cells. Mediators Inflamm 2015: 362126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JK, Keshavan MS (2011). Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxid Redox Signal 15: 2011–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalcman S, Green-Johnson JM, Murray L, Nance DM, Dyck D, Anisman H et al (1994). Cytokine-specific central monoamine alterations induced by interleukin-1, -2 and -6. Brain Res 643: 40–49. [DOI] [PubMed] [Google Scholar]
- Zalcman S, Savina I, Wise RA (1999). Interleukin-6 increases sensitivity to the locomotor-stimulating effects of amphetamine in rats. Brain Res 847: 276–283. [DOI] [PubMed] [Google Scholar]
- Zeni-Graiff M, Rizzo LB, Mansur RB, Maurya PK, Sethi S, Cunha GR et al (2016). Peripheral immuno-inflammatory abnormalities in ultra-high risk of developing psychosis. Schizophr Res176: 191–195.. [DOI] [PubMed]