Figure 3.
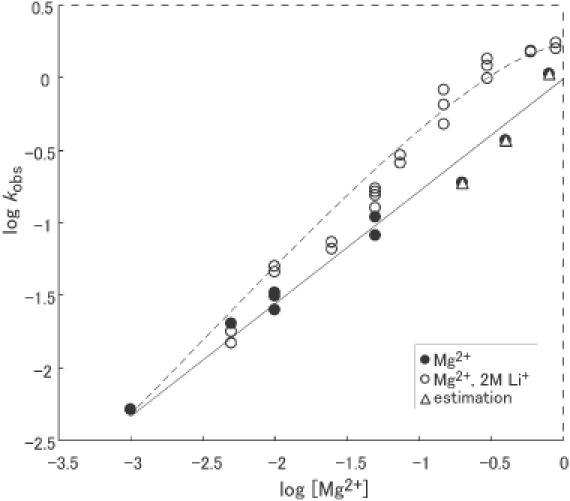
The Mg2+-titration curve for cleavage activity in the presence (open circles) and absence (filled circles) of Li+ ions. In the absence of Li+ ions, the line drawn with the linear best fit had a slope of 0.7. The activity increased with increasing concentrations of Mg2+ ions and did not reach a plateau value even at 800 mM Mg2+ ions. In the presence of 2 M Li+ ions, the activity increased with increases in the concentration of Mg2+ ions up to ∼600 mM. Triangles are the calculated rates in the presence of Mg2+ ions plus 2 M Li+ ions, which were determined simply by adding the respective reaction rates together. Since the observed rate in the presence of 2 M Li+ ions only at pH 6 was <0.01 min−1, triangles are at almost the same positions as the filled circles. The rates observed in the presence of Mg2+ ions and Li+ ions together (open circles) were clearly higher than the calculated values (triangles).
