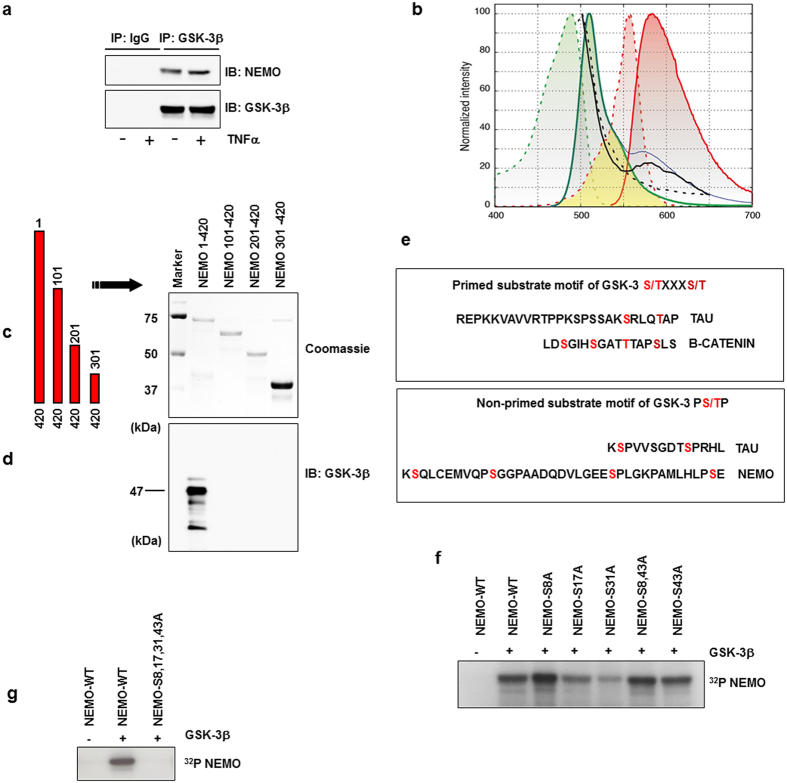
Figure 2. GSK-3β associates with human NEMO.
(a) CoIP of NEMO and GSK-3β from lysates of HEK293 cells after stimulation with TNFα (10 ng/ml) for 30 min. (b) Lambda scans of co-transfected NEMO (TagRFP) and GSK-3β (GFP) in HEK293 showed facilitated emission of NEMO-TagRFP (black curve) after excitation of GFP-GSK-3β with blue light of 476 nm by confocal laser scanning microscopy. Excitation (dotted lines) and emission (bold lines) spectra of GFP (green curves) and TagRFP (red) with the spectral overlap (yellow area) between GFP emission and TagRFP excitation showed that, theoretically, an increase of approximately 30% of TagRFP emission (blue curve) could be expected. We found an increase of approximately 20% (black curve) in our FRET experiments that could be abolished after acceptor bleaching with strong 561 nm laser light (black dotted curve). (c) Schematic representation of NEMO constructs used. (d) CoIP from lysates of HEK293 cells transfected with the constructs as indicated. Wild-type NEMO (NEMO 1-420) or NEMO deletion mutants after immunoprecipitation with GST beads (Coomassie staining). (e) Motif of primed known GSK-3 phosphorylation sites (upper box). Primed serine residues are labeled in dark red; GSK-3 phosphorylation sites are labeled in light red. Motifs for non-primed GSK-3 (lower panel) substrates are labeled in light red. NEMO serine residues labeled in light red may represent direct targets for GSK-3. (f) In vitro kinase assay using wild-type and mutant GST-NEMO fusion proteins (1 μg) as substrates for GSK-3β (0.01 μg). (g) In vitro kinase assay using wild-type and mutant GST-NEMO fusion proteins (1 μg) as substrates for GSK-3β (0.01 μg).