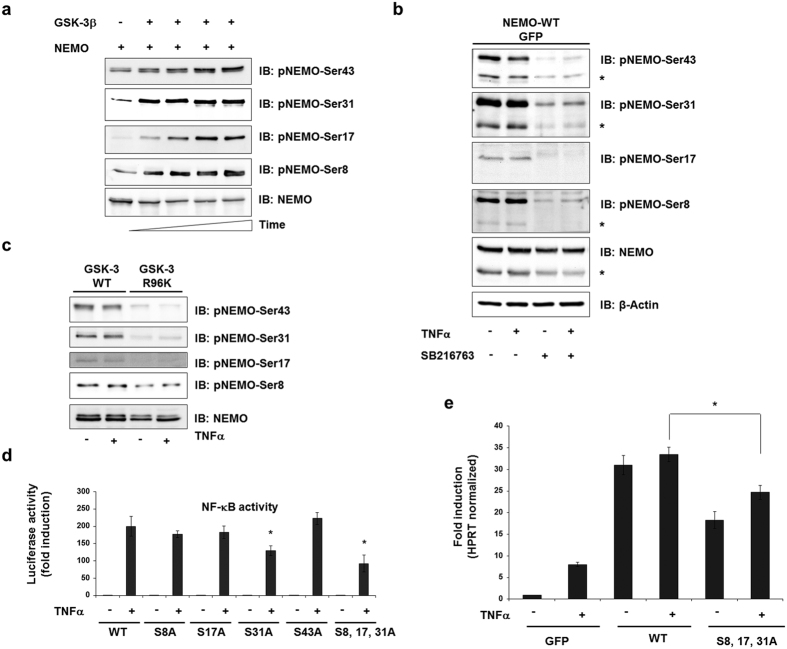
Figure 3. GSK-3β-mediated NEMO phosphorylation is required for NF-κB activity.
(a) In vitro kinase assay using recombinant human NEMO (1 μg) as substrate for GSK-3 (0.01 μg) in a time-dependent manner. The same amounts of proteins are immunoblotted with the antibodies indicated. (b) HEK293 cells were transfected wild-type NEMO. After treatment with the GSK-3 inhibitor SB216763 (1 μM) for 12 h, cells were stimulated with TNFα (10 ng/ml) for 1 h. Cell lysates were immunoblotted with the antibodies indicated. Asteriscs indicate phospho-endogenous NEMO. (c) HEK293 cells were transfected with wild-type GSK-3β or mutant GSK-3β-R96K and cell lysates were assayed for the expression of the phosphorylated form of NEMO. (d) HEK293 cells were transfected with expression vectors carrying wild-type and mutant NEMO (1 μg each), NF-κB-dependent gene expression was quantified by measuring luciferase activity. -Fold induction is the ratio of stimulated to unstimulated cells (*P < 0.05). (e) HEK293 cells were transfected either with wild-type NEMO or with mutated NEMO. The cells were treated—or not—with TNFα (10 ng/ml) for 24 h, and analyzed for the expression of NF-κB-target gene, IL8 by quantitative RT–PCR. (*P < 0.05 vs. WT).