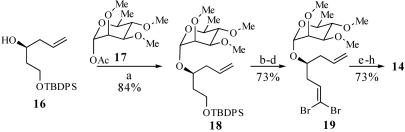
Scheme 6.
Synthesis of aldehyde 14. a, TBS trifluoromethanesulfonate, 4 Å MS, CH2Cl2, 23°C, 15 min, 84%; b, tetrabutylammonium fluoride, THF, 0°C → 23°C, 1.5 h, 84%; c, Dess–Martin periodinane, pyridine, wet CH2Cl2, 0°C → 23°C, 2.5 h, 92%; d, CBr4, Ph3P, CH2Cl2, 0°C → 23°C, 30 min, 94%; e, O3, 4:1 CH2Cl2/MeOH, KHCO3, -78°C → 23°C, 3 h; f, Ph3P CHCO2Me, CH2Cl2, 23°C, 12 h, 82% from 19, ds = 95:5; g, diisobutylaluminum hydride, CH2Cl2, -78°C → 0°C, 1.25 h, 89%; h, SO3·pyridine, DMSO, i-Pr2NEt, CH2Cl2, 0°C, 20 min.
CHCO2Me, CH2Cl2, 23°C, 12 h, 82% from 19, ds = 95:5; g, diisobutylaluminum hydride, CH2Cl2, -78°C → 0°C, 1.25 h, 89%; h, SO3·pyridine, DMSO, i-Pr2NEt, CH2Cl2, 0°C, 20 min.