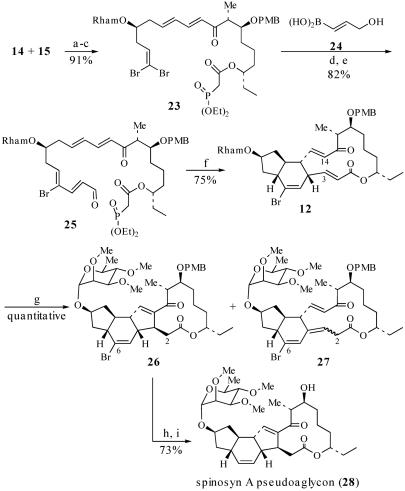
Scheme 8.
Synthesis of pseudoaglycon 28. a, Ba(OH)2, 40:1 THF/H2O, 23°C, 8 h, 93% over two steps; b, 8:8:1 THF/HOAc/H2O, 23°C, 4 h, quantitative; c, (EtO)2P(O)CH2CO2H, N-ethyl, N′-(3-dimethylaminopropyl)-carbodiimide·MeI, DMAP, CH2Cl2, 23°C, 1.5 h, 98%; d, 24, Pd(PPh3)4, Tl2CO3, 3:1 THF/H2O, 2 h, 82%; e, SO3·pyridine, DMSO, i-Pr2NEt, CH2Cl2, 0°C, 30 min; f, i-Pr2NEt, LiCl, CH3CN (1 mM), 23°C, 19 h, 75%, (E)/(Z) = ≥95:5, ds = 73:12:9:6; g, Me3P (8 eq), tert-amyl alcohol (0.005 M), 23°C, 6 h, quantitative; h, (trimethylsilyl)3SiH, AIBN, dioxane, 80°C, 1.5 h; i, DDQ, CH2Cl2/pH 7 buffer, 0°C, 3 h, 73%.