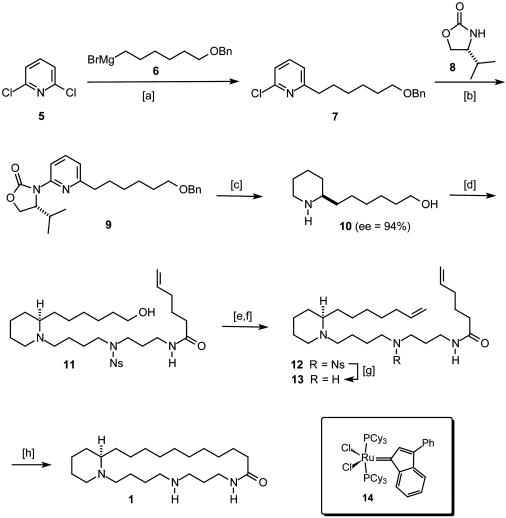
Scheme 3.
[a] Fe(acac)3 catalytic, THF/NMP, 0°C, 83%. [b] Oxazolidin-2-one 8, CuI catalytic, N,N′-dimethylethylenediamine, K2CO3, toluene, 140°C, 90%. [c] Pd(OH)2/C catalytic, MeOH, HOAc, H2 [120 atm (1 atm = 101.3 kPa)], 35°C, 78%, ee = 94%. [d] Bromide 18, K2CO3, NaI, EtOH, reflux, 73%. [e] Oxalyl chloride, DMSO, CH2Cl2, NEt3, -60°C, 81%. [f] {Ph3P-CH3}+ Br-, potassium hexamethyldisilazide, THF, HMPA, -78°C → room temperature, 82%. [g] HSCH2COOH, LiOH, DMF, 84%. [h] (i) HCl/Et2O, CH2Cl2; (ii) complex 14 catalytic, CH2Cl2, reflux; (iii) H2 (50 bars), 70°C, 76%.