Abstract
One common complication of mucopolysaccharidosis I-Hurler (MPS1-H) is corneal clouding, which occurs despite current treatments, including bone marrow transplantation. Human corneas were obtained from a 14 year old subject with MPS1-H and visual disability from progressive corneal clouding despite a prior bone marrow transplant at age 2. This was compared to a cornea from a 17 year old donated to our eye bank after his accidental death. The corneas were analyzed microscopically after staining with Alcian blue, antibodies to collagen I, IV, VI, and α-smooth muscle actin. Differences in levels of expression of the indicated molecules were assessed. Corneas from Hurler and control mice were examined similarly to determine potential mechanistic overlap. The MPS1-H subject cornea showed elevations in mucopolysaccharide deposition. The MPS1-H and Hurler mice corneas showed increased and disorganized expression of collagen I and IV relative to the control corneas. The MPS1-H corneas also showed increased and disordered expression of collagen VI. Positive expression of α-smooth muscle actin indicated myofibroblast conversion within the MPS1-H cornea in both the patient and mutant mouse material compared to normal human and control mouse cornea. Increased deposition of collagens and smooth muscle actin correlate with corneal clouding, providing a potential mechanism for corneal clouding despite bone marrow transplantation in MPS1-H patients. It might be possible to prevent or slow the onset of corneal clouding by treating the cornea with drugs known to prevent myofibroblast conversion.
Keywords: Mucopolysaccharidosis I, Hurler syndrome, Cornea, Myofibroblasts, Corneal clouding, Collagen
1. Introduction
Mucopolysaccharidosis 1-Hurler (MPS1-H) is an autosomal recessive lysosomal storage disease caused by a mutation in the gene for α-L-iduronidase. This mutation results in an abnormal accumulation of glycosaminoglycans (GAGs), ultimately resulting in severe impairment of cell structure and function. MPS1-H manifests as a multi-system disorder, with a significantly reduced lifespan if untreated. A number of ocular findings have been described in these patients, including corneal clouding, optic nerve atrophy, glaucoma, and retinopathy. One of the most common complications is corneal clouding (Ashworth et al., 2006).
Treatments of MPS1-H patients with enzyme replacement therapy or bone marrow transplantation have significantly increased life expectancy as well as quality of life for these individuals (Tolar et al., 2008; Prasad and Kurtzberg, 2010). Short term post-transplantation studies, from 6 months to almost 3 years, demonstrated that bone marrow transplantation in these patients resulted in initial clearing of the corneal clouding, reduction in optic nerve edema, and improved retinal function (Summers et al., 1989). Unfortunately, in time there can be the initiation and worsening of ocular abnormalities including corneal clouding, decreased visual function, and optic atrophy (Gullingsrud et al., 1998).
We obtained a cornea from a 14 year old patient undergoing a lamellar corneal transplant for corneal clouding 12 years after bone marrow transplantation for treatment of MPS1-H as well as an age-matched cornea. The cornea was examined for morphological changes and changes in collagen expression. Three types of collagen, each associated with a different family of collagen molecules, were analyzed. Collagen I is a fibril-forming collagen and is expressed in the cornea (Fitch et al., 1995). Collagen IV, the major collagen of basement membranes in the body, is known to be expressed in the corneal basement membrane and stroma (Kurpakus-Wheater et al., 1999), and is up-regulated in corneal scars after procedures such as radial keratotomy (Ljubimov et al., 1995). Collagen VI is a microfibrillar collagen and forms beaded filaments that play a role in tissue strength. It is thought to play an important role in the tensile strength and transparency of the corneal stroma (Cho et al., 1990). The up-regulation of collagen synthesis in the patient cornea suggested the potential for myofibroblast conversion, which was assessed by immunostaining for α-smooth muscle actin (Darby et al., 1990). All expression patterns were also assessed in the corneas of the mouse model for MPS1-H.
2. Methods
2.1. Tissue specimens
Collection of the corneal waste material was approved by all Institutional Review Boards (IRB). The use of Eye Bank tissue is exempt from IRB approval. All research complied with the tenets of the Declaration of Helsinki. Mouse tissues were collected from wild type control and from a mouse model of mucopolysaccharidosis I [MPS1-H] (Clarke et al., 1997). All mouse studies were approved by the Institutional Animal Care and Use Committee at the University of Minnesota and complied with the guidelines of the National Institutes of Health for the care and use of animals in research. Mice were obtained from a colony maintained by the Tolar laboratory, and housed by Research Animal Resources at the University of Minnesota. They were maintained in 12 h light/dark cycles and allowed to eat and drink ad libitum. Mice were sacrificed by CO2 asphyxiation, followed by exsanguination. The corneas from 5 Hurler mice, with the same mutation as the MPS1-H subject, were examined and compared to corneas from 5 wild type mice.
2.2. Patient history
The cornea specimen from the 14 year old MPS1-H patient was obtained at the time of corneal transplantation surgery and frozen until sectioned. Procurement of the corneal tissue was obtained with patient permission, and complied with the requirements of the Health Insurance Portability and Accountability Act. The control cornea was obtained from the Minnesota Lions Eye Bank from a 17 year old donor who died of accidental trauma who had no history of eye disease, was frozen, and stored at −80 °C until sectioned.
The patient was diagnosed with MPS1-H with mild corneal clouding at age 2. The patient received a successful bone marrow transplantation from an unrelated donor (100% engraftment) at age 2 years. At age 10, a decline in visual acuity and worsening of corneal clouding was documented bilaterally. She continued to have progressive decline in visual function with increase in the corneal clouding. Her visual acuity dropped to best corrected visual acuity (BCVA) of 20/250 with a thick cornea (780 μm) despite relatively normal endothelial cells on specular microscopy. After a lamellar transplant in the right eye at age 14 years, her final BCVA was 20/30 with a corneal thickness of 570 μm. The cornea of the left eye was also cloudy, but was not operated on at this time. The cornea specimen from the MPS1-H patient was frozen and stored at −80 °C until sectioned.
2.3. Histological processing
The corneas were sectioned at 10 μm in a cryostat. A minimum of 5 sections from both the MPS1 and control corneas were stained with either hematoxylin and eosin (H and E) or Alcian blue to visualize the GAG accumulations. Sections were also immunostained for collagen I, IV, and VI as well as smooth muscle actin, which has been shown to identify myofibroblasts in tissue (Darby et al., 1990). For immunohistochemical localization in the human corneas, sections were rinsed in 0.1 M phosphate buffered saline (PBS), treated with 3% H2O2 in 0.1 M phosphate buffer (PB), rinsed in PBS, blocked in 10% normal serum, followed by overnight incubation at 4 °C in antibody against one of the following: collagen I (1:1000, abcam, Cambridge, MA); collagen IV (1:1000, abcam), collagen VI (1:1000, abcam), or α-smooth muscle actin (1:50, R&D Systems, Minneapolis, MN). For examination of corneas from a mouse model of mucopolysaccharidosis I as well as control mouse corneas, the globes were dissected, frozen, and sectioned at 12 μm. They were similarly immunostained for collagens and α-smooth muscle actin. For the mouse corneas, 1:2000 dilutions were used. After a PBS rinse, the sections were incubated using the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA), and colorized by reaction with diaminobenzidine containing heavy metals. All immunostaining with a specific antibody was performed on the same day using the same reagents to ensure standardization.
2.4. Morphometric analysis
Densitometry for collagen and smooth muscle actin density was performed on 3 sections from the control and MPS1-H human corneas using the Bioquant (R and D Systems, Nashville, TN) morphometric analysis program. Multiple fields from each section were analyzed for each cornea and averaged. Data are presented as mean ± standard error of the mean.
3. Results
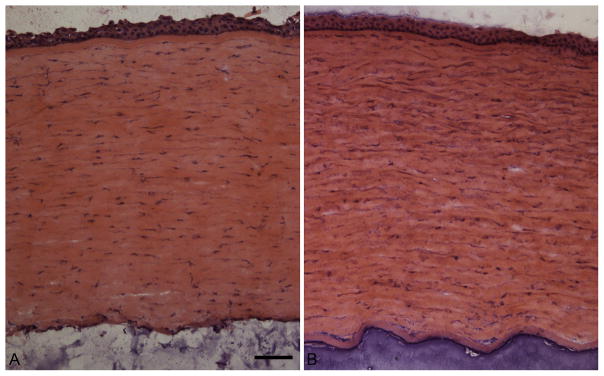
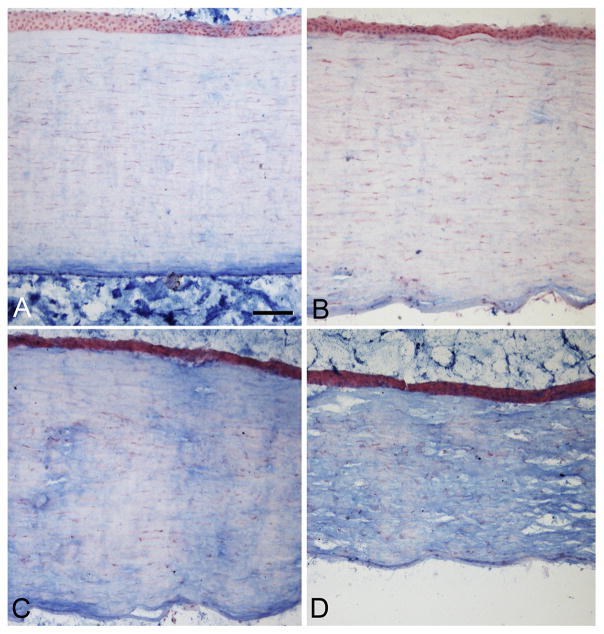
Microscopic examination of the MPS1-H cornea using standard H and E showed the stromal layer was thicker than similar regions of the normal age-matched control cornea, with a more basophilic and cellular appearance (Fig. 1). As mucopolysaccharidosis is a storage disease, we examined the corneas using Alcian blue, a staining method where the density of blue stain correlates with the amount of mucopolysaccharides in the tissues. The control cornea was relatively negative for Alcian blue staining (Fig. 2A), while the cornea from the MPS1-H subject showed a variable amount of reactivity depending on location in the cornea (Fig. 2B–D). Consistent with a prior report, these increased deposits of muco-polysaccharides were unevenly distributed (Constantopoulos et al., 1989). Similar results were seen in the corneas of the MPS1-H mouse corneas (not shown).
Fig. 1.
Hematoxylin and eosin stain of the corneas from a A) normal subject and B) a subject with mucopolysaccharidosis I (MPS1-H). Bar is 20 μm.
Fig. 2.
Alcian blue stain to visualize mucopolysaccharides in the corneal tissue. A) Normal subject. B, C, and D) Different regions of the cornea from the subject with mucopolysaccharidosis I (MPS1-H). Note the tremendous variability in intensity of stain in different regions of the patient cornea. Bar is 20 μm.
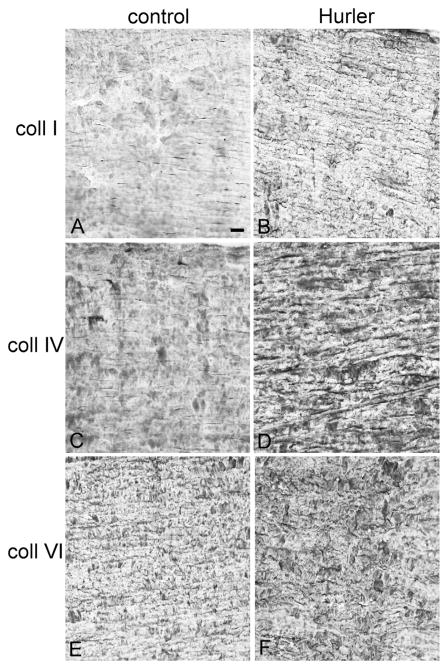
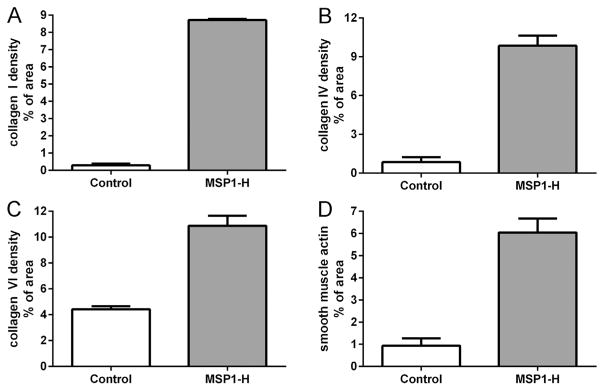
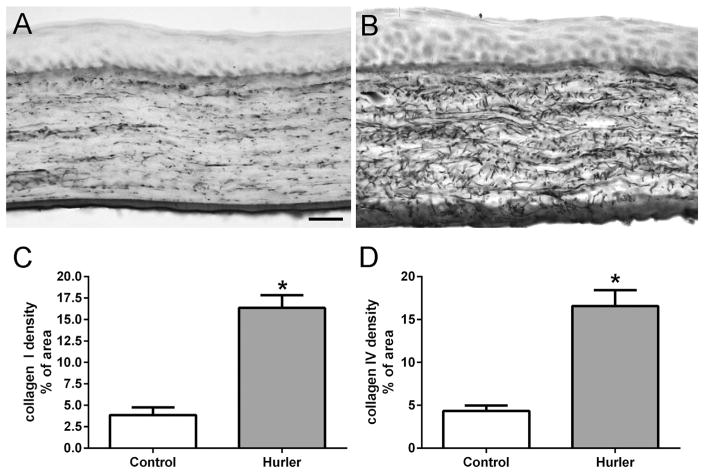
The MPS1-H patient cornea was immunostained for expression of three types of collagen, each associated with a different family of collagen molecules. Human corneas are largely type I and VI collagens (Robert et al., 2001), and so analysis of collagen staining was reflective of disorganization of the specific collagens examined. In the control human cornea, only low levels of disordered collagens I and IV were present in the stroma, with positive staining in a more orderly, linear pattern parallel to the corneal surface (Fig. 3A, C). Collagen VI was abundant in the control cornea, again in a more organized, orderly parallel array (Fig. 3E). In the cornea from the patient with MPS1-H, there were significant increases in disorganized expression levels of collagen I and IV (Fig. 3B, D). However, the majority of the staining visible was still essentially parallel to the corneal surface. There was also an increase in collagen VI immunostaining (Fig. 3F), but the pattern of expression was more disordered than in the age-matched control cornea. Collagen density was determined morphometrically (Fig. 4), and showed there was a 30-fold increase in collagen I, a 12-fold increase in collagen IV, and a 2.4-fold increase in collagen VI expression levels in the Hurler cornea, mainly due to their significant disorganization compared to the normal control. The control and Hurler mouse corneas were extremely rich in collagen I and VI. The collagen I and IV that was immunostained in the mouse corneas was linearly organized in the control corneas, but highly disorganized in the corneas of the Hurler mice (Fig. 5). Both were increased 4-fold over the control mouse corneas (Fig. 5).
Fig. 3.
Control (A, C, E) and patient (B, D, F) corneas immunostained for collagen I (A, B), collagen IV (C, D), and collagen VI (E, F). Note that for collagens I, IV, and VI there was more intense immunostaining in the cornea from the MPS1-H patient. For collagen IV and VI, the collagen fibrils appeared to be more disordered than in the control cornea. Bar is 20 μm.
Fig. 4.
Morphometric analysis of density of expression in tissue sections of human corneas from the control and MPS1-H patient immunostained for (A) collagen I, (B) collagen IV, (C) collagen VI, and (D) α-smooth muscle actin. Data were graphed as the mean ± standard error of the mean.
Fig. 5.
Immunohistochemical staining of collagen IV in (A) control mouse cornea and (B) Hurler mouse cornea. Bar is 20 μm. Morphometric analysis of density of expression in tissue sections of Hurler and control mouse corneas immunostained for (C) collagen I and (D) collagen IV.
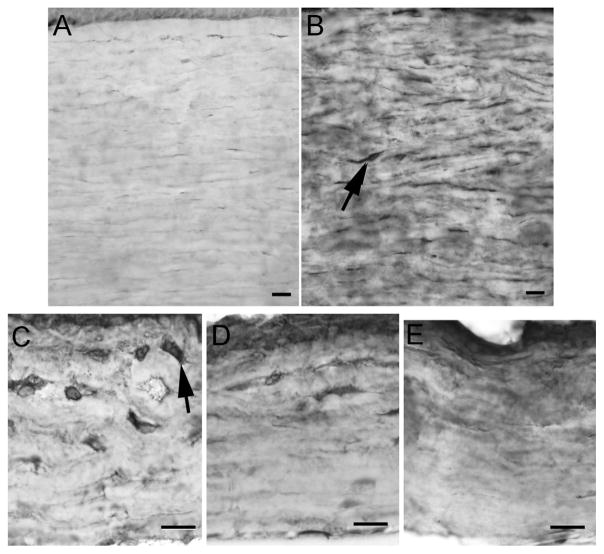
In light of the disorganized collagen changes, the corneas were examined for expression levels of α-smooth muscle actin to assay myofibroblast conversion (Figs. 4 and 6). Little to no expression of this protein was seen in the control corneas; however, there was a striking increase in expression levels of α-smooth muscle actin in the cornea from the patient with MPS1-H (Figs. 4D and 6A, B). Individual cells could be seen in the immunostained sections (arrows), suggesting a conversion of stromal cells to myofibroblasts. Density analysis showed over a 6-fold increase in the levels of α-smooth muscle actin expression in the diseased cornea. Corneas from the mouse model of MPS1-H were also examined for expression of α-smooth muscle actin (Fig. 6C–E) and compared to corneas from control mice. Increased immunostaining for α-smooth muscle actin was seen in the corneas from the mutant MPS1-H mice compared to the age-matched control corneas, and there were many enlarged individual cells presumed to be myofibroblasts (arrows) (Fig. 6C).
Fig. 6.
Control (A) and patient (B) corneas immunostained for α-smooth muscle actin. Note the significant increase in expression in the patient cornea. Arrow indicates a myofibroblast. Bar is 20 μm. (C–E) Corneas from the MPS1-H mouse model (C, D) and from a control mouse (E) immunostained for α-smooth muscle actin. Note the significant increase in expression in the patient and mutant mouse corneas. Arrows indicate myofibroblasts. Bar is 20 μm.
Using standard densitometry procedures, the density of α-smooth muscle actin was calculated based on total corneal area for the human cornea specimens (Fig. 4D). Immunostaining for α-smooth muscle actin was represented by 0.94 ± 0.33% of the area in the control cornea, whereas 6.04 ± 0.63% of the MPS1-H cornea area was positive for α-smooth muscle actin. This represents a 6-fold increase compared to the normal cornea, confirming the qualitative assessment. Collagen VI density in both the control and Hurler mouse corneas was so dense that morphometric analysis was not possible using our techniques.
4. Discussion
One of the most common ophthalmologic problems associated with MPS1-H is corneal clouding (Ashworth et al., 2006). This clouding has been attributed to the secondary alteration in the orderly packing of collagen in these corneas due to accumulation of glycosaminoglycans in the intercellular spaces (Rummelt et al., 1992). Enzyme replacement therapy does not appear to prevent the appearance or progression of corneal opacification (Pitz et al., 2007). Despite reports of stability or even short term improvement in corneal transparency after bone marrow transplantation, corneal clouding may progress at longer post-bone marrow transplantation intervals (Gullingsrud et al., 1998).
Studies have suggested that accumulated glycosaminoglycans result in disruption of the orderly collagen structure in the stroma, although without examination of specific collagen proteins. Using mainly ultrastructural approaches, the collagen fibrils were shown to have increased spacing as well as increased mean fibril diameter in the cornea from a bone marrow transplanted MPS1-H patient (Huang et al., 1996). Collagens are a large family of helical proteins, with differential expression in different body tissues, and compositional differences impact both structure and function. Collagens within the corneal stroma are a complex mixture, including collagens I, III, IV, V, an unusually high amount of collagen VI (Zimmermann et al., 1986; Delaigue et al., 1995) as well as fibril-associated (FACIT) collagens XII and XIV and other non-fibrillar collagens such as XIII and XVIII (Robert et al., 2001; Michelacci, 2003). We chose to look at collagen types I, IV, and VI, as each represents a different isoform family of collagen: fibril-forming (type I), network-forming in basement membranes (type IV), and beaded-filament forming (type VI). It is noteworthy that the expression of each of these collagens increased in the corneal stroma from the MPS1-H patient compared to the age-matched corneal control. It is not clear if this represents more collagen fibrils expressing collagen I, IV, and/or VI or if it is mainly the result of increased diameter of individual fibrils that were already present and/or increased disorganization. Future studies are needed to determine the initiating factor.
As histochemical and other evidence suggest that glycosaminoglycans play a critical role in maintaining normal collagen fibril diameter and orientation within the cornea (Borcherding et al., 1975; Rada et al., 1993), both critical for transparency of the cornea, it is not surprising that increased amounts of glycosaminoglycans in the cornea would produce clouding. However, previous studies did not examine specific collagen isoforms in corneas from patients with MPS1-H. Changes in the different collagens can be quite distinct based on the type of injury or diseases that involve the cornea. Despite collagen I being a major component of the cornea (Michelacci, 2003), collagen I changes have not been widely studied in diseased corneas or after various types of corneal injury. Collagen 1 density increased in alkali burns, after laceration, and in bullous keratopathy (Ishizaki et al., 1993; Morishige et al., 2012). We now add corneas from MPS1-H subjects to this list. Collagen IV expression is also affected in corneal injuries, and increases after corneal laceration, alkali-burns, and photorefractive keratectomy (Ishizaki et al., 1993; Winkler von Mohrenfels et al., 2002). Thus, increased collagen IV levels in MPS1-H are not surprising. Type VI collagen is a major component of the human cornea (Zimmermann et al., 1986), and is thought to play a role in controlling the orderly arrangement of collagen fibrils within the cornea. There are several possible roles that increased expression of collagen VI might play in corneal clouding in the MPS1-H cornea, including a direct impact on collagen fibril size and spacing through its binding of chondroitin/dermatan sulfate glycosaminoglycans (Nakamura et al., 1997; Takahashi et al., 1993). However, laboratory studies in vitro have shown that type VI collagen can increase corneal fibroblast and mesenchymal cell survival after injury (Howell and Doane, 1998). In a study performed in rabbits, type VI collagen was up-regulated during the healing phase after an experimental corneal injury (el-Shabrawi et al., 1998). These studies suggest that its increase has a potential “protective” function. Temporal studies in mouse MPS1-H models could address these issues, where collagen VI levels could be monitored relative to the development of corneal clouding to determine if it plays a positive or negative role.
The generation of myofibroblasts is associated with corneal injury (Jester et al., 1999). Myofibroblast conversion is associated with development of corneal haze, one example being the correlation between greater myofibroblast conversion in high myopes after either photorefractive keratectomy or laser in situ keratomileusis and subsequent corneal hazing (Mohan et al., 2003). This conversion is also associated with increased collagen synthesis within the injured cornea and corneal haze (Jester et al., 1999; Moller-Pedersen et al., 1998). This transition has been associated with a diverse array of signaling mechanisms, including but not limited to release of transforming growth factor β (TGFβ) (Jester et al., 1987), changes in stromal topography (Myrna et al., 2012), but also to changes in hyaluronan levels (Simpson et al., 2009). One working hypothesis generated by this conversion to myofibroblasts is that this transformation may be directly signaled by the increasing deposition of glycoasminoglycans in the corneas from MPS1-H patients, and this would be followed by increased collagen isoform production. Whether this is a secondary by-product caused by the keratocyte/fibroblast transformation into myofibroblasts is the subject of on-going studies in order to assess these changes temporally. This would help clarify which pathological change may be the primary pathological transformation. This observation suggests that treatments designed to prevent or slow the onset of myofibroblast transformation might increase the duration of corneal clarity in these patients and prevent increases in collagen deposition over time. These could include approaches such as inhibition of TGF-β inhibitors (Angunawela and Marshall, 2010; Milani et al., 2013) or blockade of platelet-derived growth factor (PDGF) (Kaur et al., 2009).
Bone marrow transplantation does not prevent the development of corneal clouding in MPS1-H patients. While accumulation of glycosaminoglycans and the subsequent disruption of collagen fibril organization and size have been ascribed to be the cause, an important precipitating factor may be the transformation of keratocytes into myofibroblasts, which in turn produce increased diversity and amount of collagen fibrils over time. Temporal studies are needed to address this issue, as it has therapeutic implications relative to the prevention and treatment of corneal opacification in these patients.
Acknowledgments
This work was supported by the University of Minnesota Foundation and a Grant to the Department of Ophthalmology and Visual Neurosciences from Research to Prevent Blindness Inc.
Abbreviations
- MPS1-H
mucopolysaccharidosis I-Hurler
- GAG
glycosaminoglycans
- BCVA
best corrected visual acuity
- PB
phosphate buffer
- PBS
phosphate buffered saline
Footnotes
Conflict of interest
All authors have no conflict of interest.
References
- Angunawela RJ, Marshall J. Inhibition of transforming growth factor-beta1 and its effects on human corneal fibroblasts by mannose-6-phosphate: potential for preventing haze after refractive surgery. J Cataract Refract Surg. 2010;36:121–126. doi: 10.1016/j.jcrs.2009.07.042. [DOI] [PubMed] [Google Scholar]
- Ashworth JL, Biswas S, Wraith E, Lloyd IC. The ocular features of the mucopolysaccharidoses. Eye. 2006;20:553–563. doi: 10.1038/sj.eye.6701921. [DOI] [PubMed] [Google Scholar]
- Borcherding MS, Blacik LJ, Sittig RA, Bizzell JW, Breen M, Weinstein HG. Proteoglycans and collagen fibre organization in human corneoscleral tissue. Exp Eye Res. 1975;21:59–70. doi: 10.1016/0014-4835(75)90057-3. [DOI] [PubMed] [Google Scholar]
- Cho HI, Covington HI, Cintron C. Immunolocalization of type VI collagen in developing and healing rabbit cornea. Invest Ophthalmol Vis Sci. 1990;31:1096–1102. [PubMed] [Google Scholar]
- Clarke LA, Russell CS, Pownall S, Warrington CL, Borowski A, Dimmick JE, Toone J, Jirik FR. Murine mucopolysaccharidosis type I: targeted disruption of the murine alpha-L-iduronidase gene. Hum Mol Genet. 1997;6:503–511. doi: 10.1093/hmg/6.4.503. [DOI] [PubMed] [Google Scholar]
- Constantopoulos G, Scott JA, Shull RM. Corneal opacity in canine MPS1. Invest Ophthalmol Vis Sci. 1989;30:1802–1807. [PubMed] [Google Scholar]
- Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63:21–29. [PubMed] [Google Scholar]
- Delaigue O, Arbeille B, Rossazza C, Lemesle M, Roingeard P. Quantitative analysis of immunogold labellings of collagen types I, III, IV, and VI in healthy and pathologic human corneas. Graefe’s Arch Clin Exp Ophthalmol. 1995;233:331–338. doi: 10.1007/BF00200481. [DOI] [PubMed] [Google Scholar]
- el-Shabrawi Y, Kublin CL, Cintron C. mRNA levels of α1 (VI) collagen, α1 (XII) collagen, and β1 in rabbit cornea during normal development and healing. Invest Ophthalmol Vis Sci. 1998;39:36–44. [PubMed] [Google Scholar]
- Fitch MJ, Gordon MK, Gibney EP, Linsenmayer TF. Analysis of transcriptional isoforms of collagen types IV, II, and I in the developing avian cornea by competitive polymerase chain reaction. Dev Dyn. 1995;202:42–53. doi: 10.1002/aja.1002020105. [DOI] [PubMed] [Google Scholar]
- Gullingsrud EO, Krivit W, Summers CG. Ocular abnormalities in the mucopolysaccharidoses after bone marrow transplantation. Ophthalmology. 1998;105:1099–1105. doi: 10.1016/S0161-6420(98)96014-6. [DOI] [PubMed] [Google Scholar]
- Howell SJ, Doane KJ. Type VI collagen increases cell survival and prevents anti-β1 integrin mediated apoptosis. Exp Cell Res. 1998;241:230–241. doi: 10.1006/excr.1998.4051. [DOI] [PubMed] [Google Scholar]
- Huang Y, Bron AJ, Meek KM, Vellodi A, McDonald B. Ultrastructural study of the cornea in a bone marrow-transplanted Hurler syndrome patient. Exp Eye Res. 1996;62:377–387. doi: 10.1006/exer.1996.0043. [DOI] [PubMed] [Google Scholar]
- Ishizaki M, Zhu G, Haseba T, Shafter SS, Kao WWY. Expression of collagen I, smooth muscle a-actin, and vimentin during the healing of alkali-burned and lacerated corneas. Invest Ophthalmol Vis Sci. 1993;34:3320–3328. [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- Jester JV, Rodrigues MM, Herman IM. Characterization of avascular corneal wound healing fibroblasts. New insights into the myofibroblasts. Am J Pathol. 1987;127:140–148. [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, Agrawal V, Suto C, Wilson SE. Corneal myofibroblast viability: opposing effects of IL-1 and TGF β1. Exp Eye Res. 2009;89:152–158. doi: 10.1016/j.exer.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurpakus-Wheater M, Kernacki KA, Hazlett LD. Corneal cell proteins and ocular surface pathology. Biotech Histochem. 1999;74:146–159. doi: 10.3109/10520299909047967. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Burgeson RE, Butkowski RJ, Michael AF, Sun TT, Kenney MC. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest. 1995;72:461–473. [PubMed] [Google Scholar]
- Michelacci YM. Collagens and proteoglycans of the corneal extracellular matrix. Braz J Med Biol Res. 2003;36:1037–1046. doi: 10.1590/s0100-879x2003000800009. [DOI] [PubMed] [Google Scholar]
- Milani BY, Milani FY, Park DW, Namavari A, Shah J, Armirjamshidi H, Ying H, Djalilian AR. Rapamycin inhibits the production of myofibroblasts and reduces corneal scarring after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2013;54:7424–7430. doi: 10.1167/iovs.13-12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AEK, Choi R, Hong JW, Lee LS, Mohan RR, Ambrosio R, Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Moller-Pedersen T, Li HF, Petroll WM, Cavanagh HD, Jester JV. Confocal microscopic characterization of wound repair after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 1998;39:487–501. [PubMed] [Google Scholar]
- Morishige N, Yamada N, Zhang X, Morita Y, Yamada N, Kimura K, Takahara A, Sonoda KH. Abnormalities of stromal structure in the bullous keratopathy cornea identified by second harmonic generation imaging microscopy. Invest Ophthalmol Vis Sci. 2012;53:4998–5003. doi: 10.1167/iovs.12-10214. [DOI] [PubMed] [Google Scholar]
- Myrna KE, Mendonsa R, Russell P, Pot SA, Liliensiek SJ, Jester JV, Nealey PF, Brown D, Murphy CJ. Substratum topography modulates corneal fibroblast to myofibroblast transformation. Invest Ophthalmol Vis Sci. 2012;53:811–816. doi: 10.1167/iovs.11-7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Kimura S, Kobayashi M, Hirano K, Hoshino T, Awaya S. Type VI collagen bound to collagen fibrils by chondroitin/dermatan sulfate glycosaminoglycan in mouse corneal stroma. Jpn J Ophthalmol. 1997;41:71–76. doi: 10.1016/s0021-5155(97)00011-7. [DOI] [PubMed] [Google Scholar]
- Pitz S, Ogun O, Bajbouj M, Arash L, Schulze-Frenking G, Beck M. Ocular changes in patients with mucopolysaccharidosis I receiving enzyme replacement therapy. Arch Ophthalmol. 2007;125:1353–1356. doi: 10.1001/archopht.125.10.1353. [DOI] [PubMed] [Google Scholar]
- Prasad VK, Kurtzberg J. Transplant outcomes in mucopolysaccharidoses. Semin Hematol. 2010;47:59–69. doi: 10.1053/j.seminhematol.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Rada JA, Cornuet PK, Hassell JR. Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) Exp Eye Res. 1993;56:635–648. doi: 10.1006/exer.1993.1081. [DOI] [PubMed] [Google Scholar]
- Robert L, Legeais JM, Robert AM, Renard G. Corneal collagens. Pathol Biol. 2001;49:353–363. doi: 10.1016/s0369-8114(01)00144-4. [DOI] [PubMed] [Google Scholar]
- Rummelt V, Meyer HJ, Naumann GOH. Light and electron microscopy of the cornea in systemic mucopolysaccharidosis type I-S. Cornea. 1992;11:86–92. doi: 10.1097/00003226-199201000-00014. [DOI] [PubMed] [Google Scholar]
- Simpson RML, Meran S, Thomas D, Stephens P, Bowen T, Steadman R, Phillips A. Age-related changes in pericellular hyaluronan organization leads to impaired dermal fibroblast to myofibroblasts differentiation. Am J Pathol. 2009;175:1915–1928. doi: 10.2353/ajpath.2009.090045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers CG, Purple RL, Krivit W, Pineda R, Copland GT, Ramsay NKC, Kersey JH, Whitley CB. Ocular changes in mucopolysaccharidoses after bone marrow transplantation. Ophthalmology. 1989;96:977–985. doi: 10.1016/s0161-6420(89)32795-3. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Cho HI, Kublin CL, Cintron C. Keratan sulfate and dermatan sulfate proteoglycans associate with type VI collagen in fetal rabbit cornea. J Histochem Cytochem. 1993;41:1447–1457. doi: 10.1177/41.10.8245404. [DOI] [PubMed] [Google Scholar]
- Tolar J, Grewal SS, Bjoraker KJ, Whitley CB, Shapiro EG, Charnas L, Orchard PJ. Combination of enzyme replacement and hematopoietic stem cell transplantation as a therapy for Hurler syndrome. Bone Marrow Transpl. 2008;41:531–535. doi: 10.1038/sj.bmt.1705934. [DOI] [PubMed] [Google Scholar]
- Winkler von Mohrenfels C, Reischl U, Lohmann CP. Corneal haze after photorefractive keratectomy for myopia: role of collagen IV mRNA typing as a predictor of haze. J Cataract Refract Surg. 2002;28:1446–1451. doi: 10.1016/s0886-3350(02)01273-7. [DOI] [PubMed] [Google Scholar]
- Zimmermann DR, Trueb B, Winterhalter KH, Witmer R, Fischer RW. Type VI collagen is a major component of the human cornea. FEBS Lett. 1986;3:55–58. doi: 10.1016/0014-5793(86)80297-6. [DOI] [PubMed] [Google Scholar]