Abstract
Objectives. Prolactin (PRL) is a lactation-inducing hormone with immunomodulatory properties and is found at elevated levels in the serum of patients with RA and other rheumatic diseases. The PRL receptor (PRLR) has been shown to be expressed by macrophages in atherosclerotic plaques. The aim of this study was to examine PRLR expression by synovial macrophages and its role in the regulation of macrophage activation.
Methods. Serum monomeric 23 kDa PRL levels were measured in 119 RA patients using a fluoroimmunometric assay. PRLR expression was assessed in synovial tissue of 91 RA, 15 PsA and 8 OA patients by immunohistochemistry and digital image analysis. Double IF was used to identify PRLR-expressing cells. The effects of PRL on monocyte-derived macrophage gene expression were examined by quantitative real-time PCR and ELISA.
Results. Serum PRL levels were similar in female and male RA patients. Median (interquartile range) PRLR expression was significantly higher (P < 0.05) in RA and PsA synovial tissue compared with OA. PRLR colocalized with synovial CD68+ macrophages and von Willebrand factor+ endothelial cells. In vitro, PRLR was prominently expressed in IFN-γ-and IL-10-polarized macrophages compared with other polarizing conditions. PRL by itself had negligible effects on macrophage gene expression, but cooperated with CD40L and TNF to increase expression of pro-inflammatory genes including IL-6, IL-8 and IL-12β.
Conclusions. Synovial PRLR expression is enhanced in patients with inflammatory arthritis compared with OA, and PRL cooperates with other pro-inflammatory stimuli to activate macrophages. These results identify PRL and PRLR as potential new therapeutic targets in inflammatory arthritis.
Keywords: rheumatoid arthritis, synovium, cell receptor interaction, signalling and activation, macrophages, hormones
Rheumatology key messages
Synovial prolactin receptor expression is enhanced in patients with inflammatory arthritis.
Prolactin cooperates with other pro-inflammatory stimuli to activate macrophages via engagement with the prolactin receptor.
Prolactin to prolactin receptor interaction has pro-inflammatory effects and may be a new therapeutic target in inflammatory arthritis.
Introduction
RA predominantly affects women, and it has therefore long been thought that hormones such as prolactin (PRL) and oestrogen might play an important role as cofactors in the pathogenesis of RA [1, 2]. PRL is a neuroendocrine hormone that is mainly secreted by the anterior pituitary gland, but also by cells in many extra-pituitary sites, including immune cells [3]. Its pleiotropic functions are broad, ranging from induction of lactation to influences on reproductive functions, calcium metabolism and immune reactivity [4]. In the last two decades increasing evidence has related PRL to the immune system [5, 6], supporting the concept of important interactions between the (neuro)endocrine and immune systems [7]. PRL can directly affect immune cells, as PRL can interfere with B cell tolerance induction and apoptosis of transitional B cells, which can lead to the development of autoimmunity [8]. Furthermore, PRL has an effect on the proliferation and differentiation of T cells and alters regulatory T cells to contribute to inflammation [9]. Recently, it has been shown that the PRL receptor (PRLR), which belongs to the family of haematopoietic cytokine receptors, is present in human atherosclerotic plaques at the sites of inflammation, primarily on macrophages [10]. Although the potential functional consequences of PRLR ligation on human macrophages have not been examined, PRL induces production of different cytokines, such as IL-1β, IL-12β, IFN-γ and TNF and chemokines by murine peritoneal macrophages [11, 12].
The observed decrease of disease activity in RA patients during pregnancy has been recognized for decades, possibly attributed to increase of oestrogens and progesterone [2] and a period of transient relative hypercortisolism. Conversely, breastfeeding might exacerbate disease activity in RA through the release of PRL [13], and the risk of developing RA is increased in women who are lactating after their first pregnancy [14]. The possible involvement of PRL in RA and other autoimmune diseases has also been suggested by genetic studies, as the human PRL gene locus is located on chromosome 6 close to and in linkage disequilibrium with the HLA gene loci region [15, 16]. Further supportive evidence for immune-modulatory properties is observed in animal models of arthritis, where bromocriptine, a dopamine receptor agonist that indirectly reduces PRL levels, supresses postpartum exacerbation of collagen-induced arthritis in mice [17].
Previous studies have shown elevated levels of serum PRL in RA patients compared with controls [18], both in females [19] and in males [20], although other studies have failed to confirm these findings [21]. In RA, serum PRL levels are associated with disease duration, functional stage according to the Steinbrocker classification, ESR, CRP, RF status and levels [22], and radiological progression [23]. A recent study has also shown a negative association between PRL and BMD [22]. In a limited number of small open-label clinical trials, bromocriptine has been reported to improve some clinical parameters in active RA patients [24]. Recently, it has been shown that in PsA, administration of bromocriptine improves joint and skin symptoms [25] and a case report has described amelioration of severe RA after treatment for coincidental hyperprolactinaemia with cabergoline, a dopamine receptor agonist [26]. Collectively, these findings suggest an important role for PRL in RA development and disease progression, but this has not been formally examined. Here, we examined the relationship between serum PRL levels in RA patients in relationship to disease parameters, PRLR expression in the synovial tissue (ST) of RA, PsA and OA patients, and the potential contribution of PRL to activation of the macrophages in the presence of other stimuli.
Methods
Patients and study design
Serum samples were obtained from 119 RA patients who fasted overnight and were included in a previously published study on the response to infliximab treatment [27]. All patients were diagnosed according to the 1987 ACR classification criteria for RA [28]. Patients were selected based on the availability of serum samples at baseline and 16 weeks after start of treatment. All patients had a DAS28 ⩾3.2 and were naïve to TNF inhibitors. They had failed previous treatment with ⩾2 DMARDs. Intra-articular corticosteroid injections, use of oral corticosteroids (⩽10 mg/day prednisone equivalent) and NSAIDs were allowed if dosage had been administered or stable for ⩾1 month prior to the baseline study visit.
Patients received 3 mg/kg infliximab intravenously at baseline, weeks 2 and 6 and subsequently every 8 weeks, in addition to stable dosages of MTX (5–30 mg/week). Disease duration, visual analogue scale for global disease activity (VAS GDA), tender joint count of 28 joints (TJC28), swollen joint count of 28 joints (SJC28), DAS28, HAQ score, ESR and CRP were also measured. IgM-RF and ACPA levels were determined, as measured by IgM-RF ELISA (Sanquin, Amsterdam, the Netherlands) and anti-CCP 2 ELISA (CCPlus; Euro-Diagnostica, Nijmegen, the Netherlands). Radiographs of hands and feet were obtained at baseline. Erosive disease was defined as the presence of erosions according to an experienced radiologist. Paired serum and synovial fluid (SF) samples were drawn from six RA patients with a swollen knee or ankle joint. All PsA patients fulfilled the classification criteria for psoriatic arthritis (CASPAR) [29] and active disease was defined by at least one swollen knee or ankle joint. Patients were naïve to TNF inhibitors and were treated with various DMARDs. OA patients, without history of inflammatory joint diseases, were diagnosed by an orthopaedic surgeon according to the 1986 ACR classification criteria for OA [30]. The study was performed according to the Declaration of Helsinki and approved by the Medical Ethics Committee of the Academic Medical Centre/University of Amsterdam, Amsterdam, the Netherlands. All patients gave written informed consent prior to inclusion in the study.
Fluoroimmunometric assay
Serum monomeric 23 kDa PRL levels were measured using a solid-phase, two-site, time-resolved fluoroimmunometric assay (DELFIA Prolactin, Wallac Oy, Turku, Finland). The intra-assay coefficient of variation was 4–6% (5–24 µg/l), the inter-assay coefficient of variation was 5.5–7.2% (4–50 µg/l) and the detection limit was 1.0 µg/l. All samples were assayed in duplicate in the same run. The reference values were <15 µg/l for males and <22 µg/l for females [31].
ST biopsy sampling
ST was obtained from an actively inflamed knee or ankle joint during mini-arthroscopic synovial biopsy sampling from 91 RA patients (from the same clinical study in which the serum was taken) and 15 PsA patients, as previously described [32]. Additionally, ST samples were obtained from eight OA patients during an arthroscopic procedure by an orthopaedic surgeon because of joint complaints based on degenerative disease. ST biopsy samples were immediately snap-frozen en bloc in Tissue Tek OCT (Miles, Elkhart, IN, USA). Frozen sections (5 µm) were mounted on Star Frost adhesive glass slides (Knittelgläser, Braunschweig, Germany) and stored at −80ºC until further analysis.
Immunohistochemistry
Immunohistochemistry was performed on ST sections with a primary mouse mAb against human PRLR (1A2B1; Invitrogen, Camarillo, CA, USA) using a three-step immunoperoxidase method, as previously described [33]. Further, as a negative control, irrelevant isotype-matched immunoglobulins were applied to the sections instead of the primary antibody. Two independent observers (V.C. and D.C.) unaware of the clinical data performed the semi-quantitative analysis, image acquisition and analysis. The images were analysed using a computer-assisted image analysis Syndia algorithm on a Qwin-based analysis system (Leica, Cambridge, UK), as previously described in detail [34]. Values of integrated optical density/square millimetre were obtained and corrected for the total number of nucleated cells per square millimetre, representing the intensity of staining nucleus per square millimetre [35].
IF analysis
To determine the cell types expressing PRLR, double IF was performed. ST sections were stained using the following monoclonal antibodies: anti-PRLR (1A2B1; Invitrogen, Breda, the Netherlands), anti-CD3 (SK7; Becton Dickinson, San Jose, CA, USA) for T cells, anti-CD22 (RFB4; Bioconnect, Huissen, the Netherlands) for B cells, anti-CD55 (67; Bioconnect) to detect fibroblast-like synoviocytes, anti-CD68 (Y1/82A; Biolegend, Uithoorn, the Netherlands) to detect macrophages, anti-CD138 (B-B4; Immunotech/Beckman Coulter, Woerden, the Netherlands) for plasma cells and anti-von Willebrand factor (F8/86; Dako, Glostrup, Denmark) for endothelial cells. Staining of cellular markers was performed as described previously [36]. As a negative control, irrelevant immunoglobulins were applied.
Cell isolation and macrophage stimulation
Monocytes were isolated from healthy donor buffy coats (Sanquin) using Lymphoprep (AXIS-SHIELD) density gradient centrifugation followed by Standard Isotone Percoll gradient centrifugation (GE healthcare, Amersham, Little Chalfont, UK). They were plated at 0.5 × 106 cells/ml (in total 1.5 × 106 monocytes in all polarization conditions) in Iscove’s modified Dulbecco’s medium (Invitrogen), supplemented with 1% fetal bovine serum (FBS) for 30 min at 37 °C, non-adherent cells were removed, and the monocytes were differentiated for 7 days in Iscove’s modified Dulbecco’s medium containing 10% FBS, 100 μg/ml gentamycin and 5 ng/ml GM-CSF (R&D systems, Minneapolis, MN, USA), 10 ng/ml IFN-γ (R&D systems), 10 ng/ml IL-10 (R&D systems), 25 ng/ml M-CSF (R&D systems) or 20% RA patient SF (RA SF, pooled from 5 RA patients) prior to use in experiments [37]. The SF samples were collected from patients who participated in the study based on the presence of an inflamed knee or ankle joint. The SF samples were centrifuged and stored at −20°C. Five SF samples of patients with RA were pooled prior to stimulation of the macrophages.
For cell stimulation, IFN-γ-differentiated macrophages were either left unstimulated or stimulated with soluble CD40 ligand (CD40L, 200 ng/ml, R&D systems), immunoglobulin G (IgG) beads [1:1 bead:cell ratio, cell culture grade Anti-Biotin MACSiBead Particles (Miltenyi Biotec, Bergisch Gladbach, Germany) loaded with biotinylated IgG1 (Biolegend) according to the manufacturer’s instructions at 30 µg biotinylated primary antibody per 1 × 108 bead particles], lipopolysaccharide (LPS, 1 µg/ml, Sigma-Aldrich, Taufkirchen, Germany) or TNF (10 ng/ml, Invitrogen, Camarillo, CA, USA) with or without human PRL (125 ng/ml, prepared at Inserm as previously described) [38] for 24 h. Cell-free tissue culture supernatants (after centrifugation) were harvested for cytokine analysis. IL-10-differentiated macrophages were either left unstimulated or stimulated with TNF or LPS in the presence of PRL.
RNA extraction and quantitative PCR
Total ribonucleic acid (RNA) was extracted from ST and differentiated macrophages using an RNeasy mini kit (Qiagen, Venlo, the Netherlands) and RNase-Free DNase Set (Qiagen). Further details of RNA extraction and quantitative PCR (qPCR) are detailed in the supplementary data, available at Rheumatology Online.
Ex vivo ST biopsy culture
Intact synovial biopsies from RA patients (n = 4) were cultured for 24 h in complete DMEM supplemented with 10% FBS in the absence or presence of PRL (100 ng/ml). Cell-free tissue culture supernatants were harvested and analysed for IL-6 by ELISA.
Measurement of IL-6, IL-8 and IL-12β production
Cell-free tissue culture supernatants were harvested for cytokine analysis. IL-6 and IL-8 production was measured using Pelikine Compact ELISA kit (Sanquin) and IL-12β production was measured using a DuoSet ELISA kit (R&D Systems) as per the manufacturer’s instructions.
Statistical analysis
Continuous data were described as the mean (s.d.), and as the median and interquartile range (IQR), whichever was appropriate. Wilcoxon’s signed rank test was used to analyse paired serum PRL levels of patients at baseline, and after 16 weeks and for the comparison of PRL levels in paired serum and SF. Categorical data were represented as a percentage and analysed using either the chi-square or Fisher’s exact test. The Kruskal–Wallis test was used to detect differences in synovial PRLR levels between the different study groups and the expression of the PRLR in polarized macrophages. The Mann–Whitney U test was used to analyse the serum PRL levels and PRLR expression in females and males. The data for monocyte-derived macrophages are presented as the mean (standard error of the mean) and were analysed by an overall Friedman’s test followed by post hoc Dunn’s multiple comparison test or Wilcoxon’s signed rank test, whichever was more appropriate. Correlations were assessed with Pearson’s product-moment or Spearman’s rank-order correlation coefficients, whichever was appropriate. A P-value < 0.05 was considered statistically significant. SPSS v18.0.2 software (SPSS, Chicago, IL, USA) was used for all statistical analyses.
Results
Serum PRL levels are not related to disease parameters in RA patients
The characteristics of the RA patients are summarized in Table 1. Hyperprolactinaemia (serum PRL level >15 µg/l for males and >22 µg/l for females) was observed in five (4.2%) RA patients, compared with 2–4% in the normal population [39, 40]. At baseline, the median (IQR) serum monomeric 23 kDa PRL level was 7.0 (5.0–9.5) µg/l, which was comparable (P = 0.87) to the level after 16 weeks of infliximab treatment, 7.5 (5.5–10.5) µg/l, independent of corticosteroids use or treatment response (data not shown). Paired analysis of serum at baseline compared with 16 weeks after initiation of treatment did not show a difference in PRL levels (Fig. 1A). Interestingly, PRL levels were comparable between females and males (Fig. 1B). PRL levels did not significantly correlate with disease parameters (disease duration, VAS GDA, TJC28, SJC28, ESR, CRP, DAS28, HAQ score, IgM-RF and ACPA level), independent of gender. Furthermore, serum PRL levels were similar in respect to IgM RF-positivity, ACPA-positivity and smoking status. Similar results were found in another cohort of 30 RA patients (data not shown). In a third cohort of six RA patients, we also observed no differences between serum and SF levels of PRL (Fig. 1C).
Table 1.
Patient characteristics of RA cohort in which serum prolactin levels were measured
| Characteristic | RA (n = 119) |
|---|---|
| Age, mean (s.d.), years | 55 (13) |
| Female, n (%) | 88 (74) |
| BMI, median (IQR), kg/m2 | 25.5 (23.0–27.8) |
| Current smoker, n (%) | 31 (32) |
| Disease duration, median (IQR), months | 87 (43–183) |
| Erosive disease, n (%) | 89 (75) |
| IgM-RF positive, n (%) | 84 (71) |
| IgM-RF titre, median (IQR), kU/l | 41 (7–124) |
| ACPA positive, n (%) | 84 (71) |
| ACPA titre, median (IQR), kAU/l | 165 (10–687) |
| VAS GDA, median (IQR), mm | 65 (43–77) |
| TJC28, median (IQR), n | 11 (6–17) |
| SJC28, median (IQR), n | 13 (8–17) |
| ESR, median (IQR), mm/h | 28 (13–41) |
| CRP, median (IQR), mg/l | 11.0 (4.0–23.0) |
| DAS28, median (IQR) | 5.79 (5.08–6.61) |
| Response to infliximab, n (%) | 88 (78) |
VAS GDA: visual analogue scale (range 0–100 mm) global disease activity; TJC28: tender joint count of 28 joints; SJC28: swollen joint count of 28 joints.
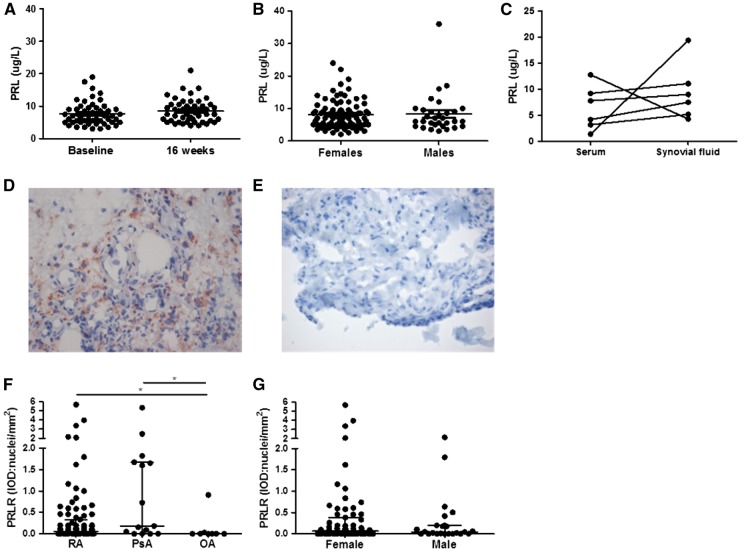
Fig. 1.
Prolactin levels in RA patients and synovial prolactin receptor expression in RA, PsA and OA patients
(A) Serum prolactin (PRL) levels were measured in paired samples obtained at baseline and after 16 weeks of infliximab treatment in 50 RA patients. (B) At baseline, serum PRL levels of 88 female RA patients were compared with 31 male RA patients. (C) PRL levels were measured in paired serum and SF from six RA patients. (D, E) Representative staining of synovial tissue with the PRL receptor (PRLR; in red) (D) or an isotype control (E) (original magnification ×40). (F) ST sections from 91 RA, 15 PsA and 8 OA patients were stained for the PRLR [integrated optical density per square millimetre (IOD): nuclei]. (G) PRLR expression in 67 female and 24 male RA patients. One dot represents an individual and the median (interquartile range) is plotted as a line in the middle. Wilcoxon’s signed-rank test was used for paired samples. The Kruskal–Wallis test where appropriate Mann–Whitney U test was used for comparison of serum PRL levels and PRLR expression. *P < 0.05.
PRLR is overexpressed in the ST of RA and PsA patients
Using immunohistochemistry, we examined the expression of the PRLR in RA, PsA and OA ST (patient characteristics are summarized in Table 2). Representative PRLR and isotype control stainings demonstrated specific staining by the anti-PRLR antibody (Fig. 1D and E). Median (IQR) synovial PRLR expression levels were significantly higher in both RA and PsA, respectively, 0.06 (0.00–0.33) and 0.18 (0.00–1.67), compared with OA, 0.00 (0.00–0.02) integrated optical density/nucleus (RA vs OA, P = 0.049; and PsA vs OA, P = 0.020) (Fig. 1F). There was no statistically significant difference in PRLR expression between females and males with RA (Fig. 1G), nor if patients were assessed independently of the disease (data not shown). There was also no correlation between the overall expression of PRLR and disease duration, VAS GDA, TJC28, SJC28, ESR or CRP levels. We used RT-PCR to detect the mRNA PRLR expression in ST of five RA, five PsA and two OA patients. The limited number of available ST samples prohibits any meaningful statistical analysis, but we did confirm PRLR expression in ST (Supplementary Fig. S1, available at Rheumatology Online).
Table 2.
Patient characteristics of the synovial tissue from the RA, PsA and OA patients
| Characteristic | RA (n = 91) | PsA (n = 15) | OA (n = 8) |
|---|---|---|---|
| Age, mean (s.d.), years | 54 (13) | 51 (16) | 64 (9) |
| Female, n (%) | 67 (74) | 8 (53) | 3 (38) |
| Disease duration, median (IQR), months | 87 (42–164) | 44 (11–85) | 129 (63–222) |
| RF positive, n (%) | 68 (75) | ND | ND |
| ACPA positive, n (%) | 67 (74) | ND | ND |
| VAS GDA, median (IQR), mm | 65 (43–77) | 58 (50–68) | ND |
| TJC28, median (IQR), n | 11 (6–17) | 3 (2–4) | ND |
| SJC28, median (IQR), n | 14 (8–19) | 2 (1–3) | ND |
| ESR, median (IQR), mm/h | 29 (16–44) | 9 (7–23) | ND |
| CRP, median (IQR), mg/l | 11.0 (4.0–27.0) | 4.0 (2.0–7.0) | ND |
| DAS28 median (IQR) | 5.80 (5.19–6.71) | 4.11 (3.23–4.33) | ND |
VAS GDA: visual analogue scale (range 0–100 mm) global disease activity; TJC28: tender joint count of 28 joints; SJC28: swollen joint count of 28 joints; ND: not determined.
ST macrophages express PRLR and macrophage PRLR expression is regulated by macrophage polarization conditions
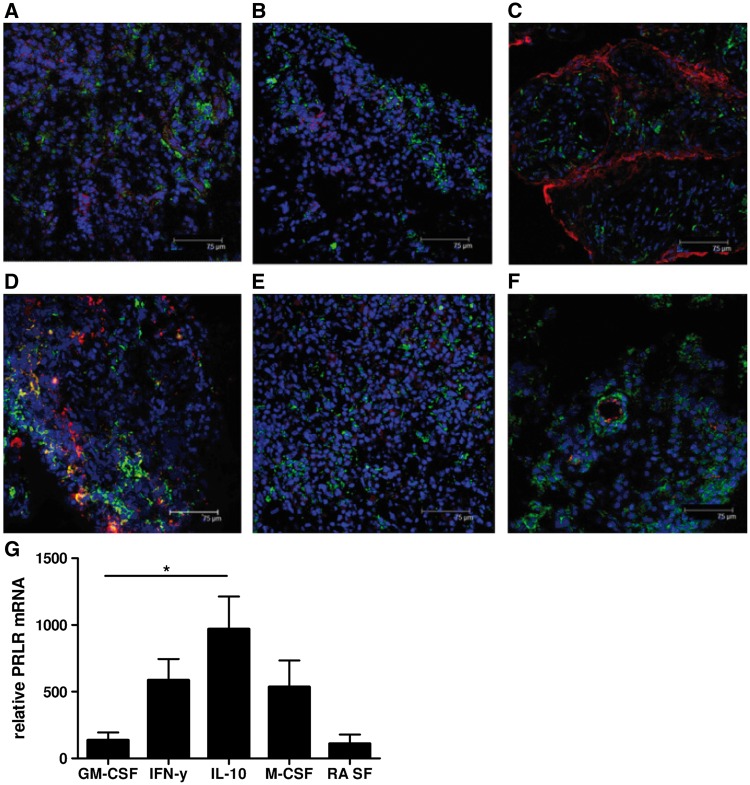
Using IF double labelling confocal microscopy, we found that PRLR expression colocalized with CD68+ macrophages and von Willebrand factor+ endothelial cells, but not with CD3+ T cells, CD22+ B cells, CD55+ fibroblast-like synoviocytes or CD138+ plasma cells (Fig. 2A–F). The presence of immunomodulatory cytokines and other factors in tissue can impact on gene expression and subsequent macrophage functional responses, a process referred to as polarization, and it has previously been shown that ST macrophages in inflammatory arthritis are phenotypically heterogeneous [41]. To determine which subtypes of macrophages express PRLR, we differentiated healthy donor monocytes under various polarizing conditions, including GM-CSF, IFN-γ, IL-10, M-CSF and RA SF. Expected phenotypes of polarized macrophages were confirmed by assessing cell surface expression patterns of CD14, CD206, CD163 and CD64 (Supplementary Fig. S2, available at Rheumatology Online). Using qPCR analysis we observed that PRLR was most predominantly expressed by macrophages differentiated in IL-10 (P < 0.05), but was also detectable under all other polarization conditions (Fig. 2G).
Fig. 2.
Prolactin receptor colocalizes with macrophages in RA and is predominantly expressed in IFN-γ- and IL-10-polarized macrophages
(A–F) Representative immunofluorescent double staining of RA ST with anti-prolactin receptor (PRLR) antibodies (green), 4',6-diamidino-2-phenylindole (DAPI) (blue) and antibodies against cellular markers (red) for T lymphocytes, CD3 (A); B lymphocytes, CD22 (B); fibroblast-like synoviocytes, CD55 (C); synovial macrophages, CD68 (D); plasma cells, CD138 (E); and vWF positive endothelial cells (F). Colocalization of PRLR was observed in CD68+ and vWF+ cells. (G) Monocyte-derived macrophages from buffy coats were differentiated using GM-CSF, IFN-γ, IL-10, M-CSF and RA SF. PRLR mRNA expression was determined in the differentiated macrophages. Data represent the mean (s.e.m.) of at least five independent experiments (except for RA SF-polarized macrophages in two independent experiments). The Kruskal–Wallis test was used. *P < 0.05.
PRLR activation in IFN-γ and IL-10-differentiated macrophages leads to dose-dependent IL-6 production
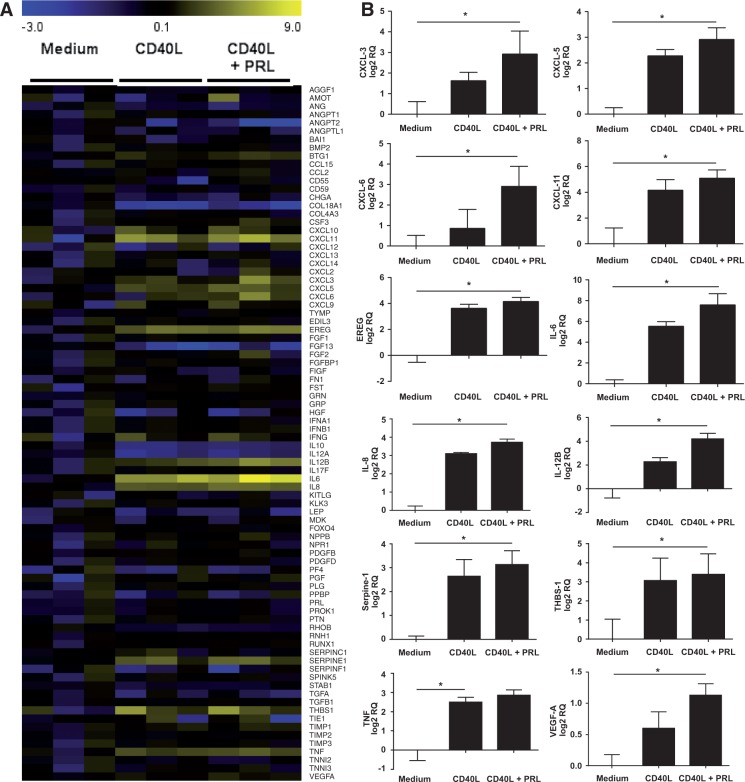
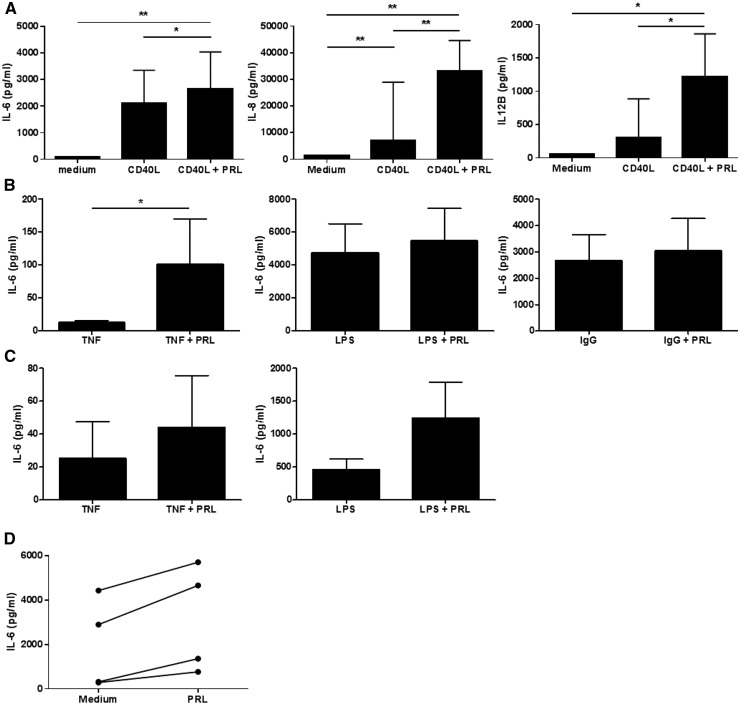
Previous studies have demonstrated that PRL can stimulate inflammatory cytokine production in murine peritoneal macrophages, but the consequences of PRLR triggering on gene expression in human macrophages have not been examined [11, 12]. In initial studies, treatment of human monocyte-derived macrophages with increasing concentrations of PRL (5, 25, 125 ng/ml) failed to stimulate macrophage production of IL-6, and failed to modulate the mRNA expression of any of 84 genes known to be involved in inflammatory and angiogenic processes (data not shown). As we had previously observed that the angiogenic receptor Tie2 has little intrinsic capacity to regulate gene expression in human macrophages, but cooperated with TNF to regulate gene expression [37], we examined the effects of PRL in combination with another inflammatory stimulus, soluble CD40L (Fig. 3A). We observed an up-regulation of the expression of several cytokines by IFN-γ-polarized macrophages, including IL-6, IL-8 and IL-12β, and chemokines, such as CXCL3, CXCL5, CXCL6 and CXCL11 after CD40L stimulation in the presence of PRL compared with CD40L alone, which did not reach statistical significance, but unlike CD40L stimulation alone, were significantly up-regulated compared with medium (Fig. 3B). These results were validated by independent qPCR analysis using different primers for IL-6, IL-8 and IL-12β (Supplementary Fig. S3, available at Rheumatology Online). We next substantiated these observations by analysing protein levels of secreted targets by ELISA. Significant increases in IL-6, IL-8 and IL-12β protein production were also observed in macrophages stimulated with CD40L and PRL, compared with CD40L alone and medium alone (P < 0.05) (Fig. 4A). Furthermore, PRL could also cooperatively boost macrophage IL-6 production in response to TNF (P < 0.05), even in a dose-dependent manner (Supplementary Fig. S4, available at Rheumatology Online), and similar trends were observed in combination with LPS (P = 0.074) and IgG (P = 0.21) (Fig. 4B). Since the PRLR is also highly expressed in IL-10-polarized macrophages, we examined the ability of PRL to cooperate with other cytokines in this phenotypically and functionally distinct sort of macrophage. In IL-10-polarized macrophages, a trend towards an increase of IL-6 production was observed after stimulation with TNF and LPS in the presence of PRL (Fig. 4C). Responsiveness to PRL was also preserved in RA synovial explants, where treatment with PRL led to increased IL-6 production in all four patients tested (Fig. 4D).
Fig. 3.
Prolactin stimulation upregulates genes involved in angiogenesis
(A) Monocyte-derived macrophages differentiated in the presence of IFN-γ (n = 3) were either left unstimulated or were stimulated with 200 ng/ml CD40L with or without 125 ng/ml prolactin (PRL). Total RNA was extracted, complementary DNA (cDNA) was synthesized and expression of 84 genes involved in angiogenesis regulation analysed using qPCR arrays and presented in a heat map. (B) Selected genes are presented as relative quantity (RQ) after log 2 transformation in unstimulated and stimulated macrophages treated with CD40L alone or in the presence of PRL (n = 3). Column bars represent the mean (s.e.m.). Data were analysed by an overall Friedman’s test followed by post hoc Dunn’s multiple comparison test. *P < 0.05.
Fig. 4.
Prolactin enhances CD40L-dependent IL-6, IL-8 and IL-12β production in macrophages and in RA synovial tissue explants
(A) Monocyte-derived macrophages differentiated in the presence of IFN-γ, were left unstimulated or stimulated with 200 ng/ml of CD40L alone or in the presence of 125 ng/ml Prolactin (PRL) for 24 h (n = 8). The mean (s.e.m.) IL-6, IL-8 and/or IL-12β concentrations (pg/ml) were analysed by using Friedman’s test. (B) IFN-γ macrophages were left stimulated with 10 ng/ml TNF-α (n = 10), 1 µg/ml LPS (n = 12) or IgG (n = 9) alone or in the presence of 125 ng/ml PRL for 24 h. IL-6 concentrations (pg/ml) were analysed by Wilcoxon’s signed rank test. (C) IL-10 macrophages were left stimulated with 10 ng/ml TNF-α (n = 8) or 1 µg/ml LPS (n = 10) alone or in the presence of 125 ng/ml PRL for 24 h. IL-6 concentrations (pg/ml) were analysed by Wilcoxon’s signed rank test. (D) Synovial biopsies from RA patients (n = 4) were cultured for 24 h in the absence or presence of PRL (100 ng/ml). IL-6 concentrations (pg/ml) are presented as dots and were analysed by Wilcoxon’s signed rank test. All cytokine levels were determined by ELISA. *P < 0.05, **P < 0.01.
Discussion
Homeostasis during inflammation is achieved by a balance between cytokines and endocrine hormones, and it is well known that (neuroendocrine) hormones play a role in auto-immune diseases [7]. However, the molecular basis for this crosstalk between the (neuro)endocrine and immune systems is not fully understood. Here, we show that PRLR is expressed by macrophages in the ST of RA and PsA patients with active disease, and that PRL, in combination with other inflammatory stimuli relevant to inflammatory arthritis, tends to enhance the expression of multiple pro-inflammatory cytokines (IL-6, IL-8, IL-12β) and chemokines (i.e. CXCL3, 5, 6 and 11) by macrophages. Previous work has shown that PRL may induce the production of TNF by murine peritoneal macrophages, consistent with a pro-inflammatory effect [12]. It has also been observed that PRL can induce the release of IL-12β in synergy with IFN-γ in murine peritoneal macrophages [42]. Others have reported that PRL, in the presence of LPS, enhances the release of haem oxygenase-1 and VEGF by human monocytes [43]. Recently, it has been shown that PRL increases TNF expression in peripheral monocytes of RA patients [44]. Our studies suggest that in polarized human macrophages, PRL by itself has little if any effect on gene expression, but it cooperates with other inflammatory stimuli present in ST to enhance cytokine and chemokine expression. These results are reminiscent of recent studies in which angiogenic Tie2 ligation on human macrophages also failed to independently regulate gene expression, but cooperated with TNF to activate both pro-inflammatory and immunoregulatory macrophages [37].
Our findings may provide in part a biological rationale for known associations between PRL and RA: disease remission during pregnancy, with higher disease activity during lactation [13] and an increased risk of developing RA after lactation [14]. During pregnancy, PRL levels increase gradually and reaching the highest levels after delivery. During pregnancy, the disease activity of RA may be controlled by an increase of oestrogens and progesterone [2] and the transient period of hypercortisolism [45], resulting in relatively less exacerbations of RA. We could not confirm a relationship between serum monomeric 23 kDa PRL levels and clinical signs and symptoms in two cohorts of RA patients (119 and 30 patients each). It is known that apart from the major isoform, monomeric 23 kDa PRL, two other isoforms (so-called big PRL and macroPRL) exist in the circulation, although only the 23 kDa PRL is predominantly biologically active [4]. It has also been shown that different assays show variable accuracy in detecting 23 kDa PRL in the presence of macroPRL [46]. Therefore, it is important in clinical cases of hyperprolactinaemia to use different assays and/or use polyethylene glycol immunoprecipitation to remove the macroPRL before measurement of PRL. Our cohort of RA patients, both male and female, was larger compared with a previously published cohort, in which 29, only male, RA patients were included [22]. The findings of the previous cohort are based on a small cohort of RA patients and therefore caution is required regarding these conclusions. This may suggest that systemic levels of PRL (originating from the pituitary) may have a minor role in the pathology of RA, while an autocrine/paracrine loop of PRL acting in the ST may be more important. Of note, an autocrine loop of PRL could enhance the inflammatory response of activated monocytes at the site of inflammation [47].
The concept of engagement of PRL with its receptor in ST (i.e. an auto-/paracrine loop) implies a potential target for therapeutic intervention. In the past, few open-label clinical trials involving small cohorts of RA and PsA patients suggested a potential clinical benefit using bromocriptine [24, 25]. In addition, the concept of locally produced PRL (over-)activating its receptor on target cells has emerged as a new mechanism in various pathological contexts, including breast and prostate cancer [48–50]. Since dopamine is inappropriate to down-regulate PRL production in extrapituitary tissues, alternative therapeutic approaches have been developed to block PRLR-mediated signalling rather that PRL production per se in target cells. PRL variants acting as competitive PRLR antagonists have been shown to efficiently down-regulate PRLR signalling, cell survival and/or proliferation in various breast or prostate cancer pre-clinical assays [49, 50]. Furthermore, a phase I clinical trial involving the treatment of breast or prostate cancer patients with PRLR-neutralizing antibodies is ongoing. If any of these drugs show efficacy in decreasing tumour size and improving clinical outcome, the use of this alternative therapeutic strategy could spark interest in the treatment of auto-immune diseases. As macrophages are known essential contributors to pathology in RA, effective targeting of macrophages expressing PRLR might provide a novel approach in the treatment of RA or other forms of inflammatory arthritis, a hypothesis that needs to be tested in well-controlled clinical trials.
Conclusions
PRL levels did not correlate with clinical signs and symptoms, and were comparable in 119 female and male RA patients. Synovial PRLR expression, which was measured by immunohistochemistry, is enhanced in patients with inflammatory arthritis compared with OA, and synovial expression of PRLR was confirmed at the mRNA level by RT-PCR. PRL cooperates with other pro-inflammatory stimuli to activate macrophages. These results identify PRL and PRLR as potential new therapeutic targets in inflammatory arthritis.
Author contributions: M.W.T. contributed to study conception and design, acquisition of the data and analysis and interpretation of the data. K.R. contributed to study conception and design, and analysis and interpretation of the data. S.G. contributed to study conception and design, acquisition of the data, and analysis and interpretation of the data. B.M.F. contributed to acquisition of the data, and analysis and interpretation of the data. V.C. contributed to study conception and design, acquisition of the data, and analysis and interpretation of the data. E.V.S. contributed to study conception and design, acquisition of the data, and analysis and interpretation of the data. V.G. contributed to study conception and design, and analysis and interpretation of the data. A.Q.R. contributed to study conception and design, and analysis and interpretation of the data. M.T.T. contributed to study conception and design, and analysis and interpretation of the data. D.M.G. contributed to study conception and design, and analysis and interpretation of the data. P.P.T. contributed to study conception and design, and analysis and interpretation of the data.
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published.
Funding: This study was sponsored by the IMI EU funded project BeTheCure no. 115142.
Disclosure statement: D.M.G. and P.P.T. are employees of GlaxoSmithKline. All other authors have declared no conflicts of interest.
Supplementary Material
Acknowledgements
We would like to thank Dr B. M. Lodde and Dr M. J. H. de Hair for editorial assistance and D. van der Coelen for his help with the digital image analysis.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Orbach H, Shoenfeld Y. Hyperprolactinemia and autoimmune diseases. Autoimmun Rev 2007;6:537–42. [DOI] [PubMed] [Google Scholar]
- 2.Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007;28:521–74. [DOI] [PubMed] [Google Scholar]
- 3.Jara LJ, Lavalle C, Fraga A. et al. Prolactin, immunoregulation, and autoimmune diseases. Semin Arthritis Rheum 1991;20:273–84. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Jonathan N, LaPensee CR, LaPensee EW. What can we learn from rodents about prolactin in humans? Endocr Rev 2008;29:1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Templ E, Koeller M, Riedl M. et al. Anterior pituitary function in patients with newly diagnosed rheumatoid arthritis. Br J Rheumatol 1996;35:350–6. [DOI] [PubMed] [Google Scholar]
- 6.Zoli A, Lizzio MM, Ferlisi EM. et al. ACTH, cortisol and prolactin in active rheumatoid arthritis. Clin Rheumatol 2002;21:289–93. [DOI] [PubMed] [Google Scholar]
- 7.Jara LJ, Medina G, Saavedra MA, Vera-Lastra O, Navarro C. Prolactin and autoimmunity. Clin Rev Allergy Immunol 2011;40:50–9. [DOI] [PubMed] [Google Scholar]
- 8.Peeva E, Michael D, Cleary J. et al. Prolactin modulates the naive B cell repertoire. J Clin Invest 2003;111:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu W, Sun M, Zhang HP. et al. Prolactin mediates psychological stress-induced dysfunction of regulatory T cells to facilitate intestinal inflammation. Gut 2014;63:1883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reuwer AQ, van EM, Houttuijn-Bloemendaal FM. et al. The prolactin receptor is expressed in macrophages within human carotid atherosclerotic plaques: a role for prolactin in atherogenesis? J Endocrinol 2011;208:107–17. [DOI] [PubMed] [Google Scholar]
- 11.Sodhi A, Tripathi A. Prolactin and growth hormone induce differential cytokine and chemokine profile in murine peritoneal macrophages in vitro: involvement of p-38 MAP kinase, STAT3 and NF-kappaB. Cytokine 2008;41:162–73. [DOI] [PubMed] [Google Scholar]
- 12.Tripathi A, Sodhi A. Prolactin-induced production of cytokines in macrophages in vitro involves JAK/STAT and JNK MAPK pathways. Int Immunol 2008;20:327–36. [DOI] [PubMed] [Google Scholar]
- 13.Barrett JH, Brennan P, Fiddler M, Silman A. Breast-feeding and postpartum relapse in women with rheumatoid and inflammatory arthritis. Arthritis Rheum 2000;43:1010–5. [DOI] [PubMed] [Google Scholar]
- 14.Silman A, Kay A, Brennan P. Timing of pregnancy in relation to the onset of rheumatoid arthritis. Arthritis Rheum 1992;35:152–5. [DOI] [PubMed] [Google Scholar]
- 15.Brennan P, Hajeer A, Ong KR. et al. Allelic markers close to prolactin are associated with HLA-DRB1 susceptibility alleles among women with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum 1997;40:1383–6. [DOI] [PubMed] [Google Scholar]
- 16.Vera-Lastra O, Jara LJ, Espinoza LR. Prolactin and autoimmunity. Autoimmun Rev 2002;1:360–4. [DOI] [PubMed] [Google Scholar]
- 17.Whyte A, Williams RO. Bromocriptine suppresses postpartum exacerbation of collagen-induced arthritis. Arthritis Rheum 1988;31:927–8. [DOI] [PubMed] [Google Scholar]
- 18.Orbach H, Zandman-Goddard G, Amital H. et al. Novel biomarkers in autoimmune diseases: prolactin, ferritin, vitamin D, and TPA levels in autoimmune diseases. Ann N Y Acad Sci 2007;1109:385–400. [DOI] [PubMed] [Google Scholar]
- 19.Ram S, Blumberg D, Newton P, Anderson NR, Gama R. Raised serum prolactin in rheumatoid arthritis: genuine or laboratory artefact? Rheumatology 2004;43:1272–4. [DOI] [PubMed] [Google Scholar]
- 20.Mateo L, Nolla JM, Bonnin MR, Navarro MA, Roig-Escofet D. High serum prolactin levels in men with rheumatoid arthritis. J Rheumatol 1998;25:2077–82. [PubMed] [Google Scholar]
- 21.Gutierrez MA, Garcia ME, Rodriguez JA. et al. Hypothalamic-pituitary-adrenal axis function in patients with active rheumatoid arthritis: a controlled study using insulin hypoglycemia stress test and prolactin stimulation. J Rheumatol 1999;26:277–81. [PubMed] [Google Scholar]
- 22.Seriolo B, Ferretti V, Sulli A, Fasciolo D, Cutolo M. Serum prolactin concentrations in male patients with rheumatoid arthritis. Ann N Y Acad Sci 2002;966:258–62. [DOI] [PubMed] [Google Scholar]
- 23.Fojtikova M, Tomasova SJ, Filkova M. et al. Elevated prolactin levels in patients with rheumatoid arthritis: association with disease activity and structural damage. Clin Exp Rheumatol 2010;28:849–54. [PubMed] [Google Scholar]
- 24.Figueroa F, Carrion F, Martinez ME. et al. [Effects of bromocriptine in patients with active rheumatoid arthritis]. Rev Med Chil 1998;126:33–41. [PubMed] [Google Scholar]
- 25.Kokot I, Pawlik-Sobecka L, Placzkowska S, Piwowar A. Prolactin as an immunomodulatory factor in psoriatic arthritis. Postepy Hig Med Dosw 2013;67:1265–72. [DOI] [PubMed] [Google Scholar]
- 26.Erb N, Pace AV, Delamere JP, Kitas GD. Control of unremitting rheumatoid arthritis by the prolactin antagonist cabergoline. Rheumatology 2001;40:237–9. [DOI] [PubMed] [Google Scholar]
- 27.Wijbrandts CA, Dijkgraaf MG, Kraan MC. et al. The clinical response to infliximab in rheumatoid arthritis is in part dependent on pretreatment tumour necrosis factor alpha expression in the synovium. Ann Rheum Dis 2008;67:1139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnett FC, Edworthy SM, Bloch DA. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 29.Taylor W, Gladman D, Helliwell P. et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- 30.Altman R, Asch E, Bloch D. et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum 1986;29:1039–49. [DOI] [PubMed] [Google Scholar]
- 31.Le MR, Endert E, Fliers E. et al. Establishment of reference values for endocrine tests. II: Hyperprolactinemia. Neth J Med 1999;55:71–5. [DOI] [PubMed] [Google Scholar]
- 32.Gerlag D, Tak PP. Synovial biopsy. Best Pract Res Clin Rheumatol 2005;19:387–400. [DOI] [PubMed] [Google Scholar]
- 33.Tak PP, van der Lubbe PA, Cauli A. et al. Reduction of synovial inflammation after anti-CD4 monoclonal antibody treatment in early rheumatoid arthritis. Arthritis Rheum 1995;38:1457–65. [DOI] [PubMed] [Google Scholar]
- 34.Haringman JJ, Vinkenoog M, Gerlag DM. et al. Reliability of computerized image analysis for the evaluation of serial synovial biopsies in randomized controlled trials in rheumatoid arthritis. Arthritis Res Ther 2005;7:R862–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Hall PO, Kraan MC, Tak PP. Quantitative image analysis of synovial tissue. Methods Mol Med 2007;135:121–43. [DOI] [PubMed] [Google Scholar]
- 36.van Kuijk AW, Wijbrandts CA, Vinkenoog M. et al. TWEAK and its receptor Fn14 in the synovium of patients with rheumatoid arthritis compared to psoriatic arthritis and its response to tumour necrosis factor blockade. Ann Rheum Dis 2010;69:301–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia S, Krausz S, Ambarus CA. et al. Tie2 signaling cooperates with TNF to promote the pro-inflammatory activation of human macrophages independently of macrophage functional phenotype. PLoS One 2014;9:e82088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paris N, Rentier-Delrue F, Defontaine A. et al. Bacterial production and purification of recombinant human prolactin. Biotechnol Appl Biochem 1990;12:436–49. [PubMed] [Google Scholar]
- 39.Alpanes M, Sanchon R, Martinez-Garcia MA, Martinez-Bermejo E, Escobar-Morreale HF. Prevalence of hyperprolactinaemia in female premenopausal blood donors. Clin Endocrinol 2013;79:545–9. [DOI] [PubMed] [Google Scholar]
- 40.El Miedany YM, Ahmed I, Moustafa H, El Baddini M. Hyperprolactinemia in Sjogren's syndrome: a patient subset or a disease manifestation? Joint Bone Spine 2004;71:203–8. [DOI] [PubMed] [Google Scholar]
- 41.Ambarus CA, Noordenbos T, de Hair MJ, Tak PP, Baeten DL. Intimal lining layer macrophages but not synovial sublining macrophages display an IL-10 polarized-like phenotype in chronic synovitis. Arthritis Res Ther 2012;14:R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majumder B, Biswas R, Chattopadhyay U. Prolactin regulates antitumor immune response through induction of tumoricidal macrophages and release of IL-12. Int J Cancer 2002;97:493–500. [DOI] [PubMed] [Google Scholar]
- 43.Malaguarnera L, Imbesi R, Di Rosa M. et al. Action of prolactin, IFN-gamma, TNF-alpha and LPS on heme oxygenase-1 expression and VEGF release in human monocytes/macrophages. Int Immunopharmacol 2005;5:1458–69. [DOI] [PubMed] [Google Scholar]
- 44.Tang C, Li Y, Lin X. et al. Prolactin increases tumor necrosis factor alpha expression in peripheral CD14 monocytes of patients with rheumatoid arthritis. Cell Immunol 2014;290:164–8. [DOI] [PubMed] [Google Scholar]
- 45.Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci 2003;997:136–49. [DOI] [PubMed] [Google Scholar]
- 46.Cavaco B, Prazeres S, Santos MA, Sobrinho LG, Leite V. Hyperprolactinemia due to big big prolactin is differently detected by commercially available immunoassays. J Endocrinol Invest 1999;22:203–8. [DOI] [PubMed] [Google Scholar]
- 47.Lopez-Rincon G, Pereira-Suarez AL, Del Toro-Arreola S. et al. Lipopolysaccharide induces the expression of an autocrine prolactin loop enhancing inflammatory response in monocytes. J Inflamm 2013;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev 2003;24:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goffin V, Bernichtein S, Touraine P, Kelly PA. Development and potential clinical uses of human prolactin receptor antagonists. Endocr Rev 2005;26:400–22. [DOI] [PubMed] [Google Scholar]
- 50.Goffin V, Hoang DT, Bogorad RL, Nevalainen MT. Prolactin regulation of the prostate gland: a female player in a male game. Nat Rev Urol 2011;8:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.