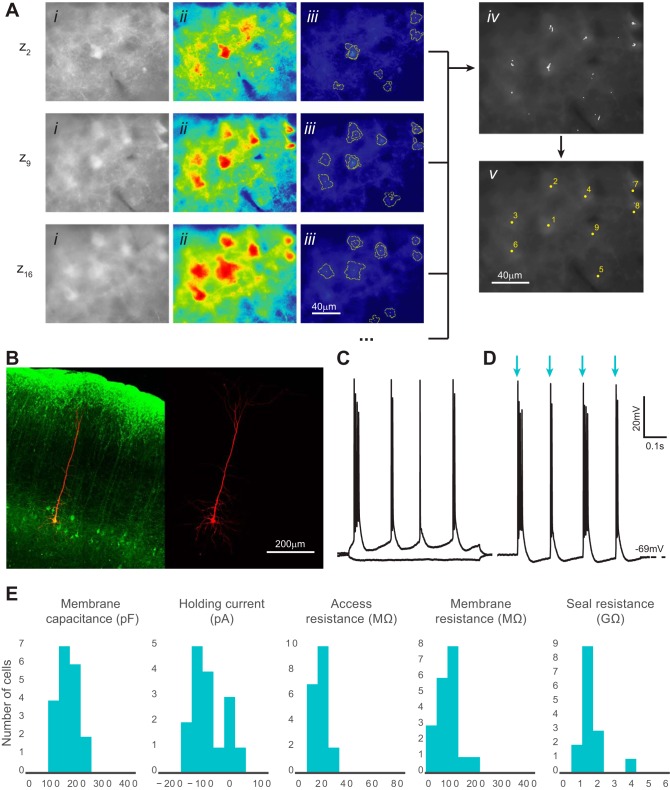
Fig. 9.
Automatic identification and patch clamp of fluorescent neurons in brain slices. A: computer vision processing of images acquired with epifluorescence optics detects fluorescent neurons and identifies their x, y, z coordinates. Three representative z sections are shown from a complete experiment (20 total z sections) using brain slices prepared from a mouse expressing channelrhodopsin-2-EYFP in layer 5 pyramidal cells (Thy1-ChR2-EYFP mouse line 18). i, Original image after histogram equalization; ii, pseudo-colored image after thresholding; iii, superimposed cell-like contours detected after a series of varying thresholds; iv, centroids of detected contours are accumulated from z sections; v, centroids from the complete z scan (20 z sections) are clustered and the final coordinates calculated. B: representative patched fluorescent neuron (green) filled with Alexa Fluor 568 dye (red) in layer 5 mouse neocortex. An acute brain slice was postfixed and immunolabeled with the anti-GFP antibody. Image acquisition was performed using confocal microscopy. C: current-clamp recordings of a patched cell responding to hyperpolarizing and depolarizing current injection. Firing pattern shows intrinsic bursting, which is characteristic of a layer 5 intrinsically bursting pyramidal neuron. D: the same neuron as in C reacts to light (480 nm) activation with bursts of action potentials. Blue arrows show the light on epochs that are 2 ms each and 150 ms apart. E: patched cell properties measured from each successful trial (n = 20 from 3 animals). No significant differences in holding current, access resistance, membrane resistance, and seal resistance were observed compared with those for nonfluorescent cells, shown in Fig. 7. Membrane capacitance distribution was significantly different from that in nonfluorescent cells, which can be explained by the larger size of the layer 5 pyramidal cells (P < 0.05, 2-tailed Student's t-test).