Abstract
More than 30 million people are infected with HIV, and HIV remains the fifth leading cause of disability-adjusted life years worldwide. Antiretroviral therapy (ART) dramatically decreases mortality rate, but there are side effects, long-term toxicities, expenses, stigmas, and inconveniences associated with chronic treatment, and HIV-infected individuals on ART have an increased risk of malignancies, cardiovascular disease, neurologic disease, and shortened life expectancy. Therefore, a cure for HIV remains an important goal. Combining new cell and gene therapy technology is an exciting approach that appears promising in vitro. Animal testing and careful clinical trials will be needed to determine if these strategies are clinically useful.
Keywords: : CCR5, cell therapy, chimeric antigen receptor, gene therapy, HIV
Introduction
More than 30 million people are infected with HIV,1 and HIV remains the fifth leading cause of disability-adjusted life years worldwide.2 Antiretroviral therapy (ART) dramatically decreases mortality rate,3 but there are side effects, long-term toxicities, expenses, stigmas, and inconveniences associated with chronic treatment, and HIV-infected individuals on ART have an increased risk of malignancies,4 cardiovascular disease,5 neurologic disease,6 and shortened life expectancy.7 Therefore, developing new HIV treatment strategies that induce long-term remission or complete eradication of HIV remains an important goal.
Long Half-life and Proliferation of HIV-Infected Cells Require New Therapies That Eradicate HIV-Infected Cells
Current antiretrovirals inhibit viral enzymes, stop viral replication, and effectively reduce plasma viral load by several logs. However, HIV-infected cells are thought to have a long half-life, on the order of 3–4 years.8,9 In addition, it has become clear that HIV-infected cells also proliferate during ART.10–13 Although many cells are infected with defective viruses, and many proviruses never reactivate, the combination of long-lived HIV-infected cells that can also proliferate makes it unrealistic that prolonged antiretrovirals alone will cure HIV simply by allowing the reservoir of HIV-infected cells to decay. Instead, new therapeutic strategies that can kill HIV-infected cells are needed. This therapeutic challenge is similar to the challenge of treating cancer. Unlike antiviral therapy, chemotherapy is designed to kill human cells with specific properties, and therefore, it seems logical to adapt therapies that have proven promising for cancer and adapt them in an effort to cure HIV. One exciting new technology is adoptive transfer of chimeric antigen receptor (CAR) expressing T cells.
Background on CAR+ T Cells for Cancer
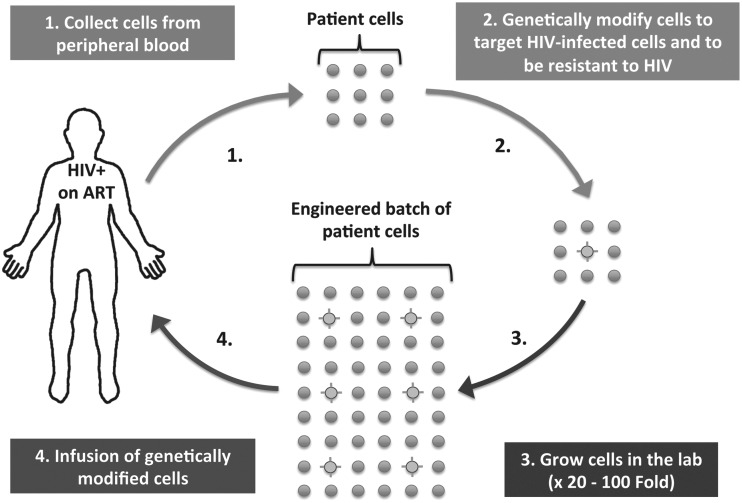
CARs are genetically engineered T cell receptors designed to redirect T cells to target cells that express specific cell-surface antigens. In most approaches, CARs are transduced into donor lymphocytes and expanded ex vivo before being transfused back into the patient (Fig. 1). CAR+ lymphocytes function by inducing MHC-independent cytotoxicity. First-generation CAR comprised an extracellular single-chain variable fragment (scFv) derived from an antibody that targets the surface of cancerous cells, linked to the intracellular domain of the T cell receptor (CD3ζ).14–19 Newer CARs include intracellular costimulatory domains (e.g., CD28 and 4-1BB), which are important for lymphocyte activation and persistence.15,16,18 Adoptive transfer of autologous lymphocytes genetically engineered with newer generation CAR has shown dramatic clinical benefit (67%, 6-month survival for relapsed/refractory leukemia compared with <25% with best available chemotherapy20), and the efficacy of the CAR+ T cells has persisted for >6 months in the majority of participants who did not undergo stem cell transplantation.20–24 Analogous to cancer, adoptive transfer of lymphocytes engineered to express anti-HIV CAR may be able to persistently target HIV-infected cells that are expressing HIV or reactive and express HIV in the future.
FIG. 1.
Schematic representation of therapy for HIV with anti-HIV CAR-expressing cells. CAR, chimeric antigen receptor.
Residual HIV Expression Despite ART is a Critical Barrier to Curing HIV
The majority of individuals on ART have no evidence of ongoing viral evolution,25–27 which argues against persistent viral replication. However, most antiretrovirals function before viral integration and do not inhibit the expression of HIV proteins from infected cells. Residual viral expression likely explains the cell-associated viral RNA,28–33 viral proteins,34–38 and low-level plasma viremia (one to three copies of HIV RNA per milliliter of blood)39–42 frequently seen during ART. Given the short half-life of free virions in the plasma,43 the plasma HIV RNA concentrations during ART imply that tens of thousands of virions are produced per day, representing a major barrier to discontinuing ART without viral rebound. Although latently infected cells clearly exist44 when ART is discontinued, high-level plasma HIV RNA normally returns within weeks,45 mirroring the timeline observed with primary infection.46 This suggests that cells actively producing virions exist and are likely an important target of efforts to cure HIV.
Mechanisms That May Allow Persistence of Residually Active HIV-Infected Cells
The paradigm has been that when long-lived latently infected cells reactive and express HIV, the HIV-infected cells are killed by cytotoxic T lymphocytes (CTLs) or direct virus-induced cell lysis.47–49 On this premise, a variety of latency-reversing agents are being investigated as a means to eliminate latently infected cells.50–55 However, Shan et al. demonstrated that HIV infection does not necessarily lead to cell death by either viral-induced cell lysis or autologous CTL-mediated effect.56 Several biological mechanisms seem to limit the efficacy of CTL-mediated clearance of reactivated cells. First, HIV evolution selects for CTL-escape mutations,57–60 which are less likely to be cleared by autologous CTL. Second, there is evidence that HIV Nef mediates downregulation of MHC-I,61–63 which helps shield HIV-infected cells from CTL responses. Third, HIV-specific CTL responses may be ineffective, either because of exhaustion64,65 or because of peripheral immune tolerance.66,67 Therefore, new strategies that circumnavigate these limitations of the host immune response, perhaps in combination with latency-reversing agents, could be important in the effort to cure HIV.
Advantages of CAR+ Lymphocytes to Target Residually Active HIV-Infected Cells
Anti-HIV CARs are appealing for three primary reasons. (1) CAR+ CTLs function independent of MHC and can therefore target HIV-infected cells that are not effectively cleared by the host's endogenous CTLs (because HIV variants evolve to escape restriction by host CTL,58–60 HIV Nef downregulates MHC-I expression,61–63 immune exhaustion,64,65 or immune tolerance66,67). (2) CAR+ lymphocytes can retain cytotoxic activity for at least 6 months,20,68,69 and CAR DNA has been detectable in the peripheral blood for up to 10 years,70 potentially providing prolonged therapeutic benefit by targeting both the actively HIV-expressing cells and cells that reactivate in the future. (3) CAR+ lymphocytes have also been found to traffic to the central nervous system,23 a potentially important reservoir of HIV, that is difficult to treat with traditional pharmacologic agents.
Previous Trials of Anti-HIV CAR
The majority of CARs have been designed to target malignant cells, but since 1991, many anti-HIV CAR strategies have been described.71–80 A Phase II randomized placebo-controlled clinical trial of a first-generation CAR to treat HIV-infected individuals on partially effective ART [only 62.5% (25/40) had viral load <50 c/mL throughout the study] demonstrated a significant decrease in infectious units per million peripheral blood mononuclear cells (−0.36 log; a commonly used measure of the viable HIV reservoir) and rectal HIV DNA (−0.5 log), and a trend toward less viral rebound, although no decrease in peripheral blood HIV DNA or rectal HIV RNA.81 Long-term follow-up suggests that this approach was safe and results in long-lived cells with CAR DNA that persisted for more than a decade.70 However, no further clinical trials of anti-HIV CAR have been reported. More recent data have revealed that HIV can infect CD8+ CAR T cells that express the CD4-zeta CAR used in the earlier trial,82,83 which may have been an important limitation of this approach.
Previous Therapy with ΔCCR5 T Cells
Another cell-based approach to treating HIV is to adoptively transfer cells that are resistant to HIV infection. The most striking example of this was the use of naturally occurring homozygous ΔCCR5 cells for stem cell transplantation, which led to the only documented HIV cure.84 However, a more practical methodology that is potentially scalable has been to collect patient cells, disrupt the CCR5 coreceptor ex vivo using a zinc finger nuclease, and then reinfuse the genetically modified cells. This approach appeared safe and feasible in a Phase I trial85 and produced a population of HIV-resistant lymphocytes (13.9% of circulating CD4 T cells 1 week after infusion), which had an estimated mean half-life of 48 weeks. Whether there was an antiviral effect with this approach was not clear from the small Phase I trial. Efforts are under way to increase the number of HIV-resistant cells, or the half-life HIV-resistant cells, in order to determine if this approach can have a clinical benefit. It remains possible that producing a population of HIV-resistant cells will restore general CD4 function and achieve a “functional cure,” but that a “sterilizing” cure will require a population of cells that are both HIV specific and HIV resistant.
Combining Cell and Gene Therapy to Treat HIV
Anti-HIV CAR T cells that are also genetically protected from HIV infection is a strategy that several groups are now pursuing. Zhen et al. introduced a short hairpin RNA upstream of a CD4-zeta CAR, which targets CCR5.83 Our group developed anti-HIV CAR T cells based on scFV from broadly neutralizing antibodies and engineered the cells to be ΔCCR5 (article in submission). In all cases, it is clear that the HIV-resistant CAR+ T cells have better antiviral activity than CAR+ T cells that are not engineered to be HIV resistant. These results demonstrate that CAR T cells can be infected by HIV and suggest that strategies which combine an HIV CAR with strategies to protect CAR+ T cells from infection might be a promising path toward a cure. As shown in Fig. 1, these therapeutic approaches are complex, highly experimental, and there is real potential for toxicity; therefore, extensive in vivo testing in animal models and carefully controlled clinical trials is needed. There is excitement that clinical studies may begin within the next few years. Despite the tantalizing promise of cell and gene therapy to treat HIV, participants who volunteer for trials will have to be carefully counseled not to assume that these approaches will be effective and they will need to clearly understand the potential risks.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Global Report: UNAIDS Report on the Global AIDS Epidemic 2012. Joint United Nations Programme on HIV/AIDS (UNAIDS). Geneva: UNAIDS, 2012. ISBN 987-92-9173-996-7 [Google Scholar]
- 2.Ortblad KF, Lozano R, Murray CJ. The burden of HIV: Insights from the Global Burden of Disease Study 2010. AIDS 2013;27:2003–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr, Baker RK, Moorman AC, et al. . Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006;43:27–34 [DOI] [PubMed] [Google Scholar]
- 4.Deeken JF, Tjen ALA, Rudek MA, et al. . The rising challenge of non-AIDS-defining cancers in HIV-infected patients. Clin Infect Dis 2012;55:1228–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Triant VA, Josephson F, Rochester CG, et al. . Adverse outcome analyses of observational data: Assessing cardiovascular risk in HIV disease. Clin Infect Dis 2012;54:408–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mothobi NZ, Brew BJ. Neurocognitive dysfunction in the highly active antiretroviral therapy era. Curr Opin Infect Dis 2012;25:4–9 [DOI] [PubMed] [Google Scholar]
- 7.Harrison KM, Song R, Zhang X. Life expectancy after HIV diagnosis based on national HIV surveillance data from 25 states, United States. J Acquir Immune Defic Syndr 2010;53:124–130 [DOI] [PubMed] [Google Scholar]
- 8.Siliciano JD, Kajdas J, Finzi D, et al. . Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 2003;9:727–728 [DOI] [PubMed] [Google Scholar]
- 9.Crooks AM, Bateson R, Cope AB, et al. . Precise quantitation of the latent HIV-1 reservoir: Implications for eradication strategies. J Infect Dis 2015;212:1361–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maldarelli F, Wu X, Su L, et al. . HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 2014;345:179–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner TA, McLaughlin S, Garg K, et al. . HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 2014;345:570–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohn LB, Silva IT, Oliveira TY, et al. . HIV-1 integration landscape during latent and active infection. Cell 2015;160:420–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simonetti FR, Sobolewski MD, Fyne E, et al. . Clonally expanded CD4+ T cells can produce infectious HIV-1 in vivo. Proc Natl Acad Sci U S A 2016;113:1883–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cartellieri M, Bachmann M, Feldmann A, et al. . Chimeric antigen receptor-engineered T cells for immunotherapy of cancer. J Biomed Biotechnol 2010;2010:956304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol 2009;21:215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipowska-Bhalla G, Gilham DE, Hawkins RE, Rothwell DG. Targeted immunotherapy of cancer with CAR T cells: Achievements and challenges. Cancer Immunol Immunother 2012;61:953–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maher J. Immunotherapy of malignant disease using chimeric antigen receptor engrafted T cells. ISRN Oncol 2012;2012:278093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilham DE, Debets R, Pule M, Hawkins RE, Abken H. CAR-T cells and solid tumors: Tuning T cells to challenge an inveterate foe. Trends Mol Med 2012;18:377–384 [DOI] [PubMed] [Google Scholar]
- 19.Turtle CJ, Hudecek M, Jensen MC, Riddell SR. Engineered T cells for anti-cancer therapy. Curr Opin Immunol 2012;24:633–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maude SL, Frey N, Shaw PA, et al. . Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochenderfer JN, Wilson WH, Janik JE, et al. . Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010;116:4099–4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grupp SA, Kalos M, Barrett D, et al. . Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brentjens RJ, Davila ML, Riviere I, et al. . CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013;5:177ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tobin NH, Learn GH, Holte SE, et al. . Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: Expression of archival virus and replication of virus. J Virol 2005;79:9625–9634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persaud D, Siberry GK, Ahonkhai A, et al. . Continued production of drug-sensitive human immunodeficiency virus type 1 in children on combination antiretroviral therapy who have undetectable viral loads. J Virol 2004;78:968–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persaud D, Ray SC, Kajdas J, et al. . Slow human immunodeficiency virus type 1 evolution in viral reservoirs in infants treated with effective antiretroviral therapy. AIDS Res Hum Retroviruses 2007;23:381–390 [DOI] [PubMed] [Google Scholar]
- 28.Fischer M, Joos B, Niederost B, et al. . Biphasic decay kinetics suggest progressive slowing in turnover of latently HIV-1 infected cells during antiretroviral therapy. Retrovirology 2008;5:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furtado MR, Callaway DS, Phair JP, et al. . Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N Engl J Med 1999;340:1614–1622 [DOI] [PubMed] [Google Scholar]
- 30.Kaiser P, Joos B, Niederost B, Weber R, Gunthard HF, Fischer M. Productive human immunodeficiency virus type 1 infection in peripheral blood predominantly takes place in CD4/CD8 double-negative T lymphocytes. J Virol 2007;81:9693–9706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasternak AO, Jurriaans S, Bakker M, Prins JM, Berkhout B, Lukashov VV. Cellular levels of HIV unspliced RNA from patients on combination antiretroviral therapy with undetectable plasma viremia predict the therapy outcome. PLoS One 2009;4:e8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yukl S, Pillai S, Li P, et al. . Latently-infected CD4+ T cells are enriched for HIV-1 Tat variants with impaired transactivation activity. Virology 2009;387:98–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmid A, Gianella S, von Wyl V, et al. . Profound depletion of HIV-1 transcription in patients initiating antiretroviral therapy during acute infection. PloS One 2010;5:e13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dybul M, Chun TW, Ward DJ, et al. . Evaluation of lymph node virus burden in human immunodeficiency virus-infected patients receiving efavirenz-based protease inhibitor—Sparing highly active antiretroviral therapy. J Infect Dis 2000;181:1273–1279 [DOI] [PubMed] [Google Scholar]
- 35.Schupbach J, Tomasik Z, Knuchel M, et al. . Optimized virus disruption improves detection of HIV-1 p24 in particles and uncovers a p24 reactivity in patients with undetectable HIV-1 RNA under long-term HAART. J Med Virol 2006;78:1003–1010 [DOI] [PubMed] [Google Scholar]
- 36.Nies-Kraske E, Schacker TW, Condoluci D, et al. . Evaluation of the pathogenesis of decreasing CD4(+) T cell counts in human immunodeficiency virus type 1-infected patients receiving successfully suppressive antiretroviral therapy. J Infect Dis 2009;199:1648–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santosuosso M, Righi E, Lindstrom V, Leblanc PR, Poznansky MC. HIV-1 envelope protein gp120 is present at high concentrations in secondary lymphoid organs of individuals with chronic HIV-1 infection. J Infect Dis 2009;200:1050–1053 [DOI] [PubMed] [Google Scholar]
- 38.Garcia MN, dos Ramos Farias MS, Åvila MM, Rabinovich RD. Presence of p24-antigen associated to erythrocyte in HIV-positive individuals even in patients with undetectable plasma viral load. PLoS One 2011;6:e14544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailey JR, Sedaghat AR, Kieffer T, et al. . Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol 2006;80:6441–6457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maldarelli F, Palmer S, King MS, et al. . ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog 2007;3:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmer S, Maldarelli F, Wiegand A, et al. . Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A 2008;105:3879–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hatano H, Delwart EL, Norris PJ, et al. . Evidence of persistent low-level viremia in long-term HAART-suppressed, HIV-infected individuals. AIDS 2010;24:2535–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Boer RJ, Ribeiro RM, Perelson AS. Current estimates for HIV-1 production imply rapid viral clearance in lymphoid tissues. PLoS Comput Biol 2010;6:e1000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henrich TJ, Hanhauser E, Marty FM, et al. . Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: Report of 2 cases. Ann Intern Med 2014;161:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davey RT, Bhat N, Yoder C, et al. . HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A 1999;96:15109–15114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen MS, Gay CL, Busch MP, Hecht FM. The detection of acute HIV infection. J Infect Dis 2010;202 Suppl 2:S270–S277 [DOI] [PubMed] [Google Scholar]
- 47.Deeks SG, Autran B, Berkhout B, et al. . Towards an HIV cure: A global scientific strategy. Nat Rev Immunol 2012;12:607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chun TW, Fauci AS. HIV reservoirs: Pathogenesis and obstacles to viral eradication and cure. AIDS 2012;26:1261–1268 [DOI] [PubMed] [Google Scholar]
- 49.Durand CM, Blankson JN, Siliciano RF. Developing strategies for HIV-1 eradication. Trends Immunol 2012;33:554–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lehrman G, Hogue IB, Palmer S, et al. . Depletion of latent HIV-1 infection in vivo: A proof-of-concept study. Lancet 2005;366:549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Contreras X, Schweneker M, Chen CS, et al. . Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol Chem 2009;284:6782–6789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science 2009;323:1304–1307 [DOI] [PubMed] [Google Scholar]
- 53.Yang HC, Xing S, Shan L, et al. . Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J Clin Invest 2009;119:3473–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xing S, Bullen CK, Shroff NS, et al. . Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation. J Virol 2011;85:6060–6064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Archin NM, Liberty AL, Kashuba AD, et al. . Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 2012;487:482–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shan L, Deng K, Shroff NS, et al. . Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 2012;36:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goulder PJ, Phillips RE, Colbert RA, et al. . Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med 1997;3:212–217 [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, McNevin JP, Holte S, McElrath MJ, Mullins JI. Dynamics of viral evolution and CTL responses in HIV-1 infection. PLoS One 2011;6:e15639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brumme ZL, John M, Carlson JM, et al. . HLA-associated immune escape pathways in HIV-1 subtype B Gag, Pol and Nef proteins. PLoS One 2009;4:e6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore CB, John M, James IR, Christiansen FT, Witt CS, Mallal SA. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science 2002;296:1439–1443 [DOI] [PubMed] [Google Scholar]
- 61.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med 1996;2:338–342 [DOI] [PubMed] [Google Scholar]
- 62.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 1998;391:397–401 [DOI] [PubMed] [Google Scholar]
- 63.Minang JT, Trivett MT, Coren LV, et al. . Nef-mediated MHC class I down-regulation unmasks clonal differences in virus suppression by SIV-specific CD8(+) T cells independent of IFN-gamma and CD107a responses. Virology 2009;391:130–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mueller YM, De Rosa SC, Hutton JA, et al. . Increased CD95/Fas-induced apoptosis of HIV-specific CD8(+) T cells. Immunity 2001;15:871–882 [DOI] [PubMed] [Google Scholar]
- 65.Petrovas C, Chaon B, Ambrozak DR, et al. . Differential association of programmed death-1 and CD57 with ex vivo survival of CD8+ T cells in HIV infection. J Immunol 2009;183:1120–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elahi S, Dinges WL, Lejarcegui N, et al. . Protective HIV-specific CD8+ T cells evade Treg cell suppression. Nat Med 2011;17:989–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kolte L, Gaardbo JC, Skogstrand K, Ryder LP, Ersboll AK, Nielsen SD. Increased levels of regulatory T cells (Tregs) in human immunodeficiency virus-infected patients after 5 years of highly active anti-retroviral therapy may be due to increased thymic production of naive Tregs. Clin Exp Immunol 2009;155:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalos M, Levine BL, Porter DL, et al. . T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 2011;3:95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kochenderfer JN, Dudley ME, Feldman SA, et al. . B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012;119:2709–2720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scholler J, Brady TL, Binder-Scholl G, et al. . Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med 2012;4:132ra153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Romeo C, Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell 1991;64:1037–1046 [DOI] [PubMed] [Google Scholar]
- 72.Roberts MR, Qin L, Zhang D, et al. . Targeting of human immunodeficiency virus-infected cells by CD8+ T lymphocytes armed with universal T-cell receptors. Blood 1994;84:2878–2889 [PubMed] [Google Scholar]
- 73.Yang OO, Tran AC, Kalams SA, Johnson RP, Roberts MR, Walker BD. Lysis of HIV-1-infected cells and inhibition of viral replication by universal receptor T cells. Proc Natl Acad Sci U S A 1997;94:11478–11483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bitton N, Verrier F, Debre P, Gorochov G. Characterization of T cell-expressed chimeric receptors with antibody-type specificity for the CD4 binding site of HIV-1 gp120. Eur J Immunol 1998;28:4177–4187 [DOI] [PubMed] [Google Scholar]
- 75.Patel SD, Moskalenko M, Tian T, et al. . T-cell killing of heterogenous tumor or viral targets with bispecific chimeric immune receptors. Cancer Gene Ther 2000;7:1127–1134 [DOI] [PubMed] [Google Scholar]
- 76.Masiero S, Del Vecchio C, Gavioli R, et al. . T-cell engineering by a chimeric T-cell receptor with antibody-type specificity for the HIV-1 gp120. Gene Ther 2005;12:299–310 [DOI] [PubMed] [Google Scholar]
- 77.Sahu GK, Sango K, Selliah N, Ma Q, Skowron G, Junghans RP. Anti-HIV designer T cells progressively eradicate a latently infected cell line by sequentially inducing HIV reactivation then killing the newly gp120-positive cells. Virology 2013;446:268–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.MacLean AG, Walker E, Sahu GK, et al. . A novel real-time CTL assay to measure designer T-cell function against HIV Env(+) cells. J Med Primatol 2014;43:341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ali A, Kitchen SG, Chen IS, Ng HL, Zack JA, Yang OO. HIV-1-specific chimeric antigen receptors based on broadly neutralizing antibodies. J Virol 2016;90:6999–7006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu B, Zou F, Lu L, et al. . CAR-T cells guided by the single-chain Fv of a broadly neutralizing antibody specifically and effectively eradicate the reactivated virus-latently-infected CD4+ T-lymphocytes isolated from HIV-1-infected individuals receiving suppressive combined antiretroviral therapy. J Virol 2016;90:9712. − 9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deeks SG, Wagner B, Anton PA, et al. . A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol Ther 2002;5:788–797 [DOI] [PubMed] [Google Scholar]
- 82.Liu L, Patel B, Ghanem MH, et al. . Novel CD4-based bispecific chimeric antigen receptor designed for enhanced anti-HIV potency and absence of HIV entry receptor activity. J Virol 2015;89:6685–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhen A, Kamata M, Rezek V, et al. . HIV-specific immunity derived from chimeric antigen receptor-engineered stem cells. Mol Ther 2015;23:1358–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hutter G, Nowak D, Mossner M, et al. . Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 2009;360:692–698 [DOI] [PubMed] [Google Scholar]
- 85.Tebas P, Stein D, Tang WW, et al. . Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med 2014;370:901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]