Abstract
Circadian clocks in eukaryotes keep time via cell-autonomous transcriptional feedback loops. A well-characterized example of such a transcriptional feedback loop is in Drosophila, where CLOCK-CYCLE (CLK-CYC) complexes activate transcription of period (per) and timeless (tim) genes, rising levels of PER-TIM complexes feed-back to repress CLK-CYC activity, and degradation of PER and TIM permits the next cycle of CLK-CYC transcription. The timing of CLK-CYC activation and PER-TIM repression is regulated posttranslationally, in part through rhythmic phosphorylation of CLK, PER, and TIM. Previous behavioral screens identified several kinases that control CLK, PER, and TIM levels, subcellular localization, and/or activity, but two phosphatases that function within the clock were identified through the analysis of candidate genes from other pathways or model systems. To identify phosphatases that play a role in the clock, we screened clock cell-specific RNA interference (RNAi) knockdowns of all annotated protein phosphatases and protein phosphatase regulators in Drosophila for altered activity rhythms. This screen identified 19 protein phosphatases that lengthened or shortened the circadian period by ≥1 hr (p ≤ 0.05 compared to controls) or were arrhythmic. Additional RNAi lines, transposon inserts, overexpression, and loss-of-function mutants were tested to independently confirm these RNAi phenotypes. Based on genetic validation and molecular analysis, 15 viable protein phosphatases remain for future studies. These candidates are expected to reveal novel features of the circadian timekeeping mechanism in Drosophila that are likely to be conserved in all animals including humans.
Keywords: Drosophila melanogaster, activity rhythms, protein phosphatases, circadian clocks
A diverse array of organisms use circadian (∼24 hr) clocks to regulate daily rhythms in physiology, metabolism, and behavior. These clocks keep time via self-sustained transcriptional feedback loops that are synchronized to daily environmental cycles and drive daily rhythms in gene expression. As in other animals, the Drosophila core timekeeping loop is activated by two bHLH‐PAS transcription factors, CLOCK and CYCLE (CLK-CYC), and repressed by PERIOD-TIMELESS (PER-TIM) complexes. The generation of self-sustaining oscillations depends on posttranslational regulation of clock proteins, which modulates their stability, activity, and/or subcellular localization during the daily cycle. Multiple levels of posttranslational controls are built into this oscillatory system to produce a ∼24 hr period, support a robust cycling amplitude, and enable resetting by environmental inputs.
The best characterized posttranslational modification of clock proteins is phosphorylation. In Drosophila, nuclear localization of the PER-TIM repressor complex is regulated by CASEIN KINASE II (CK2) and SHAGGY (SGG) phosphorylation (Martinek et al. 2001; Lin et al. 2002; Akten et al. 2003, 2009), whereas PER degradation in the nucleus is regulated by DOUBLE-TIME (DBT) and NEMO (Kloss et al. 1998, 2001; Price et al. 1998; Chiu et al. 2008, 2011). Phosphorylation also controls CLK-CYC function where NEMO, DBT, and CK2-dependent phosphorylation control CLK stability and activity (Kim and Edery 2006; Yu et al. 2006; Szabo et al. 2013). The phosphorylation state of a protein is controlled dynamically by protein kinases and phosphatases. However, few phosphatases have been identified that function in the Drosophila circadian clock; Protein Phosphatase 2a (PP2a) and Protein Phosphatase 1 (PP1), control PER-TIM repressor stability and nuclear localization (Sathyanarayanan et al. 2004; Fang et al. 2007), and the PP2a-STRIPAK complex dephosphorylates CLK to promote CLK-CYC transcription (Andreazza et al. 2015). Despite our understanding of how phosphorylation controls nuclear localization and degradation of PER-TIM repressor complexes and activity of CLK-CYC activator complexes, the role phosphorylation plays in controlling cytoplasmic PER-TIM accumulation and the light-dependent TIM degradation are not well understood. These events control progression through the feedback loop, and are therefore critical for controlling the period, phase, and amplitude of rhythmic transcription.
To determine how dephosphorylation regulates rhythmic transcription within the Drosophila clock, we used RNAi knockdown to screen all annotated protein phosphatases in Drosophila for defects in locomotor activity rhythms. Of 86 protein phosphatase or protein phosphatase regulator genes screened, 19 showing period alterations or arrhythmicity were identified as candidate clock protein phosphatases, including LEUKOCYTE ANTIGEN-LIKE (LAR), which is required to build a neuronal circuit in the Drosophila brain that mediates circadian activity rhythms (Agrawal and Hardin 2016). Additional genetic reagents were obtained or generated to validate the RNAi phenotypes. The validated phosphatases identified here may contribute to clock cell development or the circadian timekeeping mechanism, and represent potential genetic links to clock-associated disorders in humans and novel targets for the development of drugs to treat such disorders.
Materials and Methods
Fly stocks
The w1118 and w1118;CyO/Sco;TM2/TM6B strains were used as wild-type controls for activity rhythms and as balancers to generate lines used for screening and analysis, respectively. The following Gal4 strains were used to drive RNAi expression in clock cells: w1118;timGal462, w1118;;pdfGal4, and w1118;;timGal416. The following RNAi strains from the Vienna Drosophila RNAi Center (VDRC) were used to knockdown phosphatase/regulator expression in clock cells, listed as the gene name and abbreviation or CG number followed by the VDRC line number in parenthesis: puckered, puc (GD3018); Protein tyrosine phosphatase 52F, Ptp52F (GD3116); TbCMF46 (GD17123); string, stg (GD17760); CG17124 (GD19078); Calcineurin B, CanB (GD21611); Protein phosphatase 19C, Pp4-19C (GD25317); Protein tyrosine phosphatase 4E, Ptp4E (GD27232); Calcineurin A1, CanA1 (GD32283); CG17598 (GD32956); Protein phosphatase 1 at 87B, Pp1-87B (GD35025); Phosphotyrosyl phosphatase activator, Ptpa (GD41912); microtubule star, mts (GD41924); sds22 (GD42051); Mitogen-activated protein kinase phosphatase 3, Mkp3 (GD45415); Protein phosphatase 2A at 29B, Pp2a-29B (GD49671); CG6380 (KK100121); CG17746 (KK100178); CG2104 (KK100216); Mapmodulin (KK100283); Glycogen binding subunit 70E, Gbs-70E (KK100593); CG42327 (KK100914); Ppm1 (KK101257); CG10376 (KK101335); widerborst, wdb (KK101406); Calcium and integrin binding family member 2, Clb2 (KK101474); Inhibitor-2, I-2 (KK101547); Protein phosphatase V, PpV (KK101997); Protein phosphatase Y at 55A, PpY-55A (KK102021); Protein phosphatase N at 58A, PpN-58A (KK102060); CG14297 (KK102071); Protein tyrosine phosphatase 36E, Ptp36E (KK102397); CG31469 (KK102474); Glycogen binding subunit 76A, Gbs-76A (KK103044); Protein phosphatase 2B at 14D, Pp2B-14D (KK103144); CG32568 (KK103317); CG7115 (KK103354); Cep97 (KK103357); cell division cycle 14, cdc14 (KK103627); Protein tyrosine phosphatase Meg, Ptpmeg (KK103740); CG32812 (KK104081); twins, tws (KK104167); Protein phosphatase D6, PpD6 (KK104211); MAP kinase-specific phosphatase, Mkp (KK104374); Protein tyrosine phosphatase Meg2, Ptpmeg2 (KK104427); Protein phosphatase D5, PpD5 (KK104452); flapwing, flw (KK104677); CG11597 (KK104729); Protein tyrosine phosphatase 69D, Ptp69D (KK104761); Protein Tyrosine Phosphatase mitochondrial 1, PTPMT1 (KK104774); Dullard, Dd (KK104785); myopic, mop (KK104860); MAPK Phosphatase 4, MKP-4 (KK104884); CG13197 (KK105122); Protein phosphatase 2C, Pp2C (KK105249); Protein phosphatase 4 regulatory subunit 2-related protein, PPP4R2r (KK105399); alphabet, alph (KK105483); CG15528 (KK105484); Protein phosphatase 1α at 96A, Pp1α-96A (KK105525); inhibitor-t, I-t (KK105565); CG6036 (KK105568); CG5026 (KK105674); CG7378 (KK106098); CG10417 (KK106180); TFIIF-interacting CTD phosphatase, Fcp1 (KK106253); PP2A-B’ (KK107057); Protein phosphatase D3, PpD3 (KK107386); CG4733 (KK107621); Protein phosphatase 1 at 13C, Pp1-13C (KK107770); Leukocyte-antigen-related-like, Lar (KK107996); slingshot, ssh (KK107998); eyes absent, eya (KK108071); corkscrew, csw (KK108352); Protein tyrosine phosphatase 99A, Ptp99A (KK108505); CG10089 (KK108744); CG8509 (KK108802); Nuclear inhibitor of Protein phosphatase 1, NIPP1 (KK108859); Protein tyrosine phosphatase 61F, Ptp61F (KK108888); Protein phosphatase 1, Y-linked 2, PP1-Y2 (KK109147); CG14411 (KK109622); Calcineurin A at 14F, CanA-14F (KK109858); CG3632 (KK110167); PH domain leucine-rich repeat protein phosphatase, Phlpp (KK110360); Protein tyrosine phosphatase 10D, Ptp10D (KK110443); IA-2 protein tyrosine phosphatase, IA-2 (KK110595); and CG3530 (KK110786). The following strains were used to characterize candidate clock protein phosphatases: UAS-mtsRNAi (GD35171), w1118;;UAS-mts, w1118;P{EP}Pp2A-29BEP2332, w1118;P{RS3}Pp2A-29BCB-5426-3, w1118;PBac{WH}CG17746f05041, y1w*;P{EP}CG17746G4827, y1w1118;PBac{3HPy+}I-2C362, w*;P{UAS-I-2.HA}G/+;P{UAS-Pp1-87B.HA}1H, y1w67c23;P{SUPor-P}CG7115KG02655, w1118;UAS-Cep97, UAS-Cep97/Y, w1118;;UAS-Cep97, y1;P{SUPor-P}tocKG08989PpD6KG08989, y1w*Mi{MIC}Ptpmeg2MI03011/Y, w67c23P{lacW}Ptpmeg2G0232/Y, y1w67c23P{Mae-UAS.6.11}Ptpmeg2GG01129/Y, w1118PBac{WH}Ptpmeg2f06600, w*;;Ptp69D1, w*;;Df(3L)8ex25, w1118;;Ptp69D10, w1118;;Ptp69D18, w1118;;Ptp69D20, w1118;;Ptp69D21, w1118;;UAS-Ptp69D, w1118;;UAS-DNPtp69D, y1P{SUPor-P}MKP-4KG03420, w1118;;P{GSV6}Pp1α-96AGS11179, w1118;;Pp1α-96A2, w1118;;UAS-Pp1α-96A.HA, w1118;;Pp1α96A-CRISPRmutant-1/+, w1118;;Pp1α96A-CRISPRmutant-2/+, w1118;;Pp1α96A-CRISPRmutant-3/+, w1118;UAS-PP2C-like, w*;Lar13.2/+, Df(2L)TW84,l(2)74i1,amosTftLarTW84/+, Df(2L)E55,rdo1hook1LarE55pr1/+, w1118;;UAS-Lar, w1118;UAS-CanA-14Fmyc, w1118;;UAS-CanA-14Fact-myc, CanA-14F-KO/Y, and UAS-CG3530RNAi (GD26216). Although Dicer 2 enhances the transgenic RNAi effect in ∼50% of the lines tested (Dietzl et al. 2007), we chose not to coexpress Dicer 2 because of the increased off-target effects and lethality that may result.
Drosophila activity monitoring and behavior analysis
One to three d old male flies were entrained for 3 d in 12:12 light-dark (LD) and transferred to constant darkness (DD) for 7 d at 25°. The screen employed testing of each UAS-RNAi alone (as a control), driver alone (as a control), and a combination of UAS-RNAi line with timGal4 or pdfGal4 (as the RNAi knockdown). Locomotor activity was monitored using the Drosophila Activity Monitor (DAM) system (Trikinetics). Analyses of period, power and rhythm strength during DD was carried out using ClockLab (Actimetrics) software as previously described (Pfeiffenberger et al. 2010). UAS-RNAi lines that produce consistent period lengthening or shortening of ≥1 hr with p ≤ 0.05 compared to UAS-RNAi and Gal4 driver controls or >50% arrhythmicity were analyzed further. Different genetic backgrounds may show small differences in circadian period, therefore a period change of ≤1 hr due to RNAi knockdown was not considered significant.
Generation of Pp1α-96A CRISPR mutants
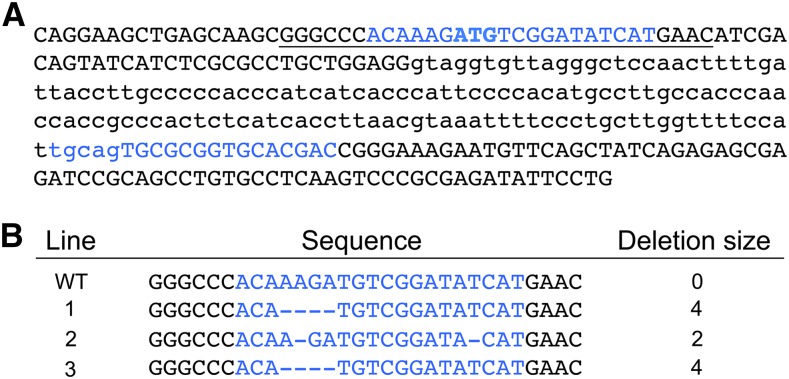
The CRISPR-Cas9 system was used to generate deletions in Pp1α-96A (Gratz et al. 2015). Two guide RNAs (gRNAs) targeting the Pp1α-96A translation start and intron 1 splice acceptor sequences, 5′-ATGATATCCGACATCTTTGT-3′ and 5′-TGCAGTGCGCGGTGCACGAC-3′, were designed using the CRISPR database (http://www.flyrnai.org/crispr2/). Complementary oligonucleotides corresponding to these gRNAs were annealed and inserted into the U6-BK-gRNA vector for expression in Drosophila (Ren et al. 2013). The resulting Pp1α-96A gRNA plasmids were sequenced to confirm the integrity of the gRNA inserts (Gene Technologies Laboratory, Texas A&M University). Pp1α-96A gRNA plasmids were then sent for injection into y1 M{vas-Cas9}ZH2A w1118 embryos (Best Gene Inc.), which express Cas9 in the germ line. Injected embryos that survived to adulthood were crossed with w1118;CyO/Sco;TM2/TM6B, and once progeny were observed, the injected adults were screened for deletions between or flanking the gRNAs. To screen for deletions, a ∼600 bp DNA fragment containing the gRNA binding sites was amplified using primers 5′-TGACCAAAGGGCGAGATTAG-3′ and 5′-ACATAGTCGCCCAGGAACAG-3′ via PCR, and sequenced. In a screen of ∼150 injected adults, three independent deletion mutants were recovered (Figure 1).
Figure 1.
Generation of Pp1α-96A mutants using the CRISPR-Cas9 system. (A) Nucleotide sequence of the Pp1α-96A genomic region targeted for mutagenesis. Capital letters, exon sequence; lowercase letters, intron sequence; blue letters, gRNA targets; bold letters, ATG translation start codon; underlined sequence, region shown in panel (B). (B) Nucleotide sequences of control wild-type (WT) and three Pp1α-96A mutant lines. The deleted nucleotides listed are shown as dashes in the sequence. CRISPR, clustered regularly interspaced short palindromic repeats; gRNA, guide RNA.
Testing for the presence of perSLIH mutation
To determine whether flies carry the perSLIH allele, a DNA fragment containing the perSLIH mutation site was amplified from genomic DNA using the Per-F (5′-GCGCTGCTCTGAATGAATCCGG-3′) and Per-R (5′-ATTGCTCACTCGTTTCCAGGACC-3′) primers via PCR. These DNA fragments were sequenced using the primer 5′- CTCCGGCAGCAGTGGCTATG-3′ to determine whether they contained a C to A nucleotide change at position 3035 corresponding to the perSLIH mutation (Hamblen et al. 1998).
Isogenizing phosphatase alleles to w1118
The w*;;Ptp69D1 and CanA14F-KO null mutants are both marked with w+, and were isogenized by backcrossing w+ male progeny to the w1118 reference strain (BDSC) for seven generations.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
To identify phosphatases that mediate circadian clock function, clock cell-specific RNAi knockdowns of all annotated Drosophila protein phosphatases were screened for altered locomotor activity rhythms. An RNAi screening strategy is advantageous because: (1) lethality can be avoided by targeting RNAi to clock cells via the Gal4/UAS system, (2) RNAi is efficient method for knocking down target gene expression, and (3) transgenic UAS-RNAi lines are available for all annotated protein phosphatase/regulator from either the VDRC, Transgenic RNAi Project at the Harvard Medical School plans (TRiP), or the National Institute of Genetics RNAi Stocks (NIG-Fly). RNAi lines from VDRC were used in this screen because the vast majority are inserted at a single genomic site (e.g., KK lines) that affords efficient and comparable expression, though some lines used were inserted at random genomic sites (e.g., GD lines) because KK lines for some protein phosphatases/regulators were not available. A total of 94 annotated protein phosphatase catalytic and regulatory subunits are present in the Drosophila genome (Drosophila RNAi Screening Center, DRSC). When this screen was initiated in 2010, we were able to obtain UAS-RNAi lines for 67 of these protein phosphatase catalytic and regulatory subunits and an additional 19 protein phosphatase regulators. Thus, a total of 86 protein phosphatases/regulators were screened to identify phosphatases that disrupt circadian activity rhythms.
Flies bearing UAS-RNAi and the w1118;timGal462 driver or controls bearing UAS-RNAi alone and Gal4 driver alone were placed into Drosophila Activity Monitors, entrained for 3 d in LD cycles, and then released into DD for 7 d. Locomotor activity rhythms recorded during DD were analyzed for circadian rhythmicity and period via ClockLab software. This initial screen identified a total of 26 candidate phosphatases, all of which lengthened or shortened circadian period by ≥1 hr or were ≥50% arrhythmic (Table 1). Since many RNAi lines with a lengthened period also had a low proportion of rhythmic flies, we were concerned that the widespread RNAi knockdown of these phosphatases or ectopic RNAi expression from the w1118;timGal462 driver impaired fly health. Consequently, we used w1118;;pdfGal4 to restrict RNAi expression to PDF neuropeptide-expressing ventral lateral pacemaker neurons (LNvs) and another timGal4 insert, w1118;;timGal416, to drive protein phosphatase/regulator RNAi expression. Out of the 86 lines screened with all three Gal4 drivers, 19 RNAi lines with a significantly (p ≤ 0.05 compared to controls) different period or >50% arrhythmic were identified (Table 1).
Table 1. Activity rhythms of clock cell-specific phosphatase RNAi knockdowns in Drosophila.
| Genotype | N | % Rhythmic | Period Mean ± SEM |
|---|---|---|---|
| w1118;+/GD3018 (puc) | 13 | 100 | 23.57 ± 0.08 |
| w1118;timGal4/GD3018 | 16 | 100 | 24.40 ± 0.05 |
| w1118;GD 3018/+;timGal4/+ | 15 | 93 | 24.21 ± 0.16 |
| w1118;GD3018/+;pdfGal4/+ | 15 | 100 | 24.13 ± 0.06 |
| w1118;+/GD3116 (Ptp52F) | 13 | 92 | 23.92 ± 0.15 |
| w1118;timGal4/GD3116 | 16 | 94 | 24.50 ± 0.09 |
| w1118;GD3116/+;timGal4/+ | 9 | 78 | 24.36 ± 0.22 |
| w1118;GD3116/+;pdfGal4/+ | 8 | 75 | 23.92 ± 0.18 |
| w1118;+/GD17123 (TbCMF46) | 16 | 94 | 23.5 |
| w1118;timGal4/GD17123 | 16 | 81 | 24.08 ± 0.14 |
| w1118;GD17123/+;timGal4/+ | 15 | 100 | 24.00 ± 0.12 |
| w1118;GD17123/+;pdfGal4/+ | 16 | 88 | 23.89 ± 0.13 |
| w1118;+/GD17760 (stg) | 16 | 100 | 23.56 ± 0.04 |
| w1118;timGal4/GD17760 | 16 | 94 | 24.10 ± 0.11 |
| w1118;GD17760/+;timGal4/+ | 14 | 100 | 24.11 ± 0.14 |
| w1118;GD17760/+;pdfGal4/+ | 13 | 85 | 23.86 ± 0.15 |
| w1118;+/GD19078 (CG17124) | 16 | 100 | 23.50 ± 0.06 |
| w1118;timGal4/GD19078 | 16 | 100 | 23.81 ± 0.10 |
| w1118;GD19078/+;timGal4/+ | 16 | 88 | 24.36 ± 0.09 |
| w1118;GD19078/+;pdfGal4/+ | 12 | 100 | 24.04 ± 0.11 |
| w1118;+/GD21611 (CanB) | 16 | 100 | 23.03 ± 0.03 |
| w1118;timGal4/GD21611 | 15 | 93 | 24.64 ± 0.14 |
| w1118;GD21611/+;timGal4/+ | 14 | 100 | 24.14 ± 0.16 |
| w1118;GD21611/+;pdfGal4/+ | 16 | 100 | 23.66 ± 0.09 |
| w1118;+/GD25317 (Pp4-19C) | 14 | 100 | 23.71 ± 0.08 |
| w1118;timGal4/GD25317 | 14 | 100 | 24.93 ± 0.09 |
| w1118;GD25317/+;timGal4/+ | 15 | 93 | 24.39 ± 0.15 |
| w1118;GD25317/+;pdfGal4/+ | 16 | 100 | 23.72 ± 0.09 |
| w1118;+/GD27232 (Ptp4E) | 16 | 100 | 23.50 ± 0.08 |
| w1118;timGal4/GD27232 | 16 | 100 | 24.41 ± 0.05 |
| w1118;GD27232/+;timGal4/+ | 8 | 75 | 24.50 ± 0.20 |
| w1118;GD27232/+;pdfGal4/+ | 13 | 100 | 24.54 ± 0.88 |
| w1118;+/GD32283 (CanA1) | 16 | 100 | 23.50 ± 0.04 |
| w1118;timGal4/GD32283 | 14 | 93 | 24.39 ± 0.23 |
| w1118;GD32283/+;timGal4/+ | 16 | 94 | 24.53 ± 0.10 |
| w1118;GD32283/+;pdfGal4/+ | 14 | 93 | 24.00 ± 0.13 |
| w1118;+/GD32956 (CG17598) | 16 | 94 | 23.90 ± 0.10 |
| w1118;timGal4/GD32956 | 15 | 93 | 24.54 ± 0.09 |
| w1118;GD32956/+;timGal4/+ | 14 | 43 | 24.58 ± 0.08 |
| w1118;GD32956/+;pdfGal4/+ | 16 | 94 | 24.20 ± 0.08 |
| w1118;+/GD35025 (Pp1-87B) | 15 | 100 | 23.83 ± 0.10 |
| w1118;timGal4/GD35025 | 16 | 100 | 24.63 ± 0.08 |
| w1118;GD35025/+;timGal4/+ | 13 | 100 | 24.23 ± 0.12 |
| w1118;GD35025/+;pdfGal4/+ | 16 | 100 | 24.34 ± 0.12 |
| w1118;+/GD41912 (Ptpa) | 16 | 100 | 23.69 ± 0.09 |
| w1118;timGal4/GD41912 | 15 | 100 | 24.57 ± 0.04 |
| w1118;GD41912/+;timGal4/+ | 15 | 80 | 24.04 ± 0.15 |
| w1118;GD41912/+;pdfGal4/+ | 11 | 100 | 24.23 ± 0.16 |
| w1118;+/GD41924 (mts) | 16 | 100 | 23.53 ± 0.03 |
| w1118;timGal4/GD41924 | 16 | 88 | 25.14 ± 0.15 |
| w1118;GD41924/+;timGal4/+ | 11 | 100 | 24.73 ± 0.08 |
| w1118;GD41924/+;pdfGal4/+ | 14 | 93 | 24.73 ± 0.08 |
| w1118;+/GD42051 (sds22) | 14 | 86 | 23.71 ± 0.09 |
| w1118;timGal4/GD42051 | 16 | 100 | 24.81 ± 0.06 |
| w1118;GD42051/+;timGal4/+ | 16 | 94 | 24.87 ± 0.11 |
| w1118;GD42051/+;pdfGal4/+ | 14 | 100 | 24.14 ± 0.12 |
| w1118;+/GD45415 (Mkp3) | 15 | 93 | 23.78 ± 0.09 |
| w1118;timGal4/GD45415 | 15 | 100 | 24.67 ± 0.08 |
| w1118;GD45415/+;timGal4/+ | 15 | 80 | 24.33 ± 0.15 |
| w1118;GD45415/+;pdfGal4/+ | 14 | 93 | 24.38 ± 0.10 |
| w1118;+/GD49671 (Pp2a-29B) | 15 | 100 | 23.70 ± 0.06 |
| w1118;timGal4/GD49671 | 30 | — | — |
| w1118;GD49671/+;timGal4/+ | 15 | 100 | 26.13 ± 0.09 |
| w1118;GD49671/+;pdfGal4/+ | 16 | 94 | 26.17 ± 0.20 |
| w1118;+/KK100121 (CG6380) | 16 | 100 | 23.63 ± 0.07 |
| w1118;timGal4/KK100121 | 14 | 29 | 23.38 ± 0.13 |
| w1118;KK100121/+;timGal4/+ | 9 | 0 | AR |
| w1118;KK100121/+;pdfGal4/+ | 15 | 7 | 23.5 |
| w1118;+/KK100178 (CG17746) | 16 | 88 | 23.43 ± 0.05 |
| w1118;timGal4/KK100178 | 12 | 0 | AR |
| w1118;KK100178/+;timGal4/+ | 12 | 33 | 25.88 ± 0.32 |
| w1118;KK100178/+;pdfGal4/+ | 12 | 0 | AR |
| w1118;+/KK100216 (CG2104) | 16 | 94 | 23.5 |
| w1118;timGal4/KK100216 | 16 | 63 | 23.55 ± 0.05 |
| w1118;KK100216/+;timGal4/+ | 15 | 53 | 23.75 ± 0.15 |
| w1118;KK100216/+;pdfGal4/+ | 14 | 100 | 23.61 ± 0.06 |
| w1118;+/KK100283 (Mapmodulin) | 15 | 100 | 23.07 ± 0.06 |
| w1118;timGal4/KK100283 | 16 | 81 | 23.89 ± 0.21 |
| w1118;KK100283/+;timGal4/+ | 14 | 93 | 24.04 ± 0.18 |
| w1118;KK100283/+;pdfGal4/+ | 16 | 100 | 23.78 ± 0.11 |
| w1118;+/KK100593 (Gbs-70E) | 16 | 100 | 23.53 ± 0.03 |
| w1118;timGal4/KK100593 | 13 | 54 | 25.21 ± 0.33 |
| w1118;KK100593/+;timGal4/+ | 16 | 88 | 25.46 ± 0.17 |
| w1118;KK100593/+;pdfGal4/+ | 13 | 8 | 23.5 |
| w1118;+/KK100914 (CG42327) | 16 | 100 | 23.53 ± 0.03 |
| w1118;timGal4/KK100914 | 16 | 100 | 23.78 ± 0.11 |
| w1118;KK100914/+;timGal4/+ | 15 | 80 | 23.5 |
| w1118;KK 100914/+;pdfGal4/+ | 16 | 100 | 23.53 ± 0.03 |
| w1118;+/KK101257 (Ppm1) | 28 | 89 | 23.50 ± 0.06 |
| w1118;timGal4/KK101257 | 15 | 20 | 24.67 ± 0.44 |
| w1118;KK101257/+;timGal4/+ | 13 | 23 | 24.67 ± 0.17 |
| w1118;KK101257/+;pdfGal4/+ | 15 | 40 | 23.42 ± 0.08 |
| w1118;+/KK101335 (CG10376) | 16 | 100 | 23.5 |
| w1118;timGal4/KK101335 | 16 | 94 | 24.30 ± 0.12 |
| w1118;KK101335/+;timGal4/+ | 16 | 88 | 24.32 ± 0.53 |
| w1118;KK101335/+;pdfGal4/+ | 16 | 88 | 23.75 ± 0.17 |
| w1118;+/KK101406 (wdb) | 15 | 87 | 23.42 ± 0.05 |
| w1118;timGal4/KK101406 | 16 | 88 | 24.20 ± 0.09 |
| w1118;KK101406/+;timGal4/+ | 15 | 53 | 25.86 ± 0.23 |
| w1118;KK101406/+;pdfGal4/+ | 13 | 92 | 23.83 ± 0.09 |
| w1118;+/KK101474 (Clb2) | 16 | 100 | 23.69 ± 0.09 |
| w1118;timGal4/KK101474 | 16 | 88 | 23.43 ± 0.16 |
| w1118;KK101474/+;timGal4/+ | 16 | 88 | 23.61 ± 0.14 |
| w1118;KK101474/+;pdfGal4/+ | 14 | 100 | 23.57 ± 0.05 |
| w1118;+/KK101547 (I-2) | 16 | 94 | 23.47 ± 0.03 |
| w1118;timGal4/KK101547 | 14 | 43 | 25.25 ± 0.35 |
| w1118;KK101547/+;timGal4/+ | 16 | 31 | 25.40 ± 0.17 |
| w1118;KK101547/+;pdfGal4/+ | 13 | 23 | 24.83 ± 0.59 |
| w1118;+/KK101997 (PpV) | 16 | 100 | 23.44 ± 0.04 |
| w1118;timGal4/KK101997 | 12 | 50 | 23.50 ± 0.20 |
| w1118;KK101997/+;timGal4/+ | 12 | 67 | 24.81 ± 0.34 |
| w1118;KK101997/+;pdfGal4/+ | 10 | 0 | AR |
| w1118;+/KK102021 (PpY-55A) | 16 | 100 | 23.47 ± 0.03 |
| w1118;timGal4/KK102021 | 15 | 93 | 24.21 ± 0.17 |
| w1118;KK102021/+;timGal4/+ | 14 | 64 | 23.78 ± 0.18 |
| w1118;KK102021/+;pdfGal4/+ | 14 | 100 | 23.89 ± 0.18 |
| w1118;+/KK102060 (PpN-58A) | 28 | 96 | 23.50 ± 0.03 |
| w1118;timGal4/KK102060 | 15 | 60 | 24.06 ± 0.18 |
| w1118;KK102060/+;timGal4/+ | 15 | 60 | 24.39 ± 0.14 |
| w1118;KK102060/+;pdfGal4/+ | 13 | 92 | 23.92 ± 0.18 |
| w1118;+/KK102071 (CG14297) | 25 | 100 | 23.53 ± 0.05 |
| w1118;timGal4/KK102071 | 15 | 67 | 23.55 ± 0.17 |
| w1118;KK102071/+;timGal4/+ | 16 | 75 | 24.00 ± 0.19 |
| w1118;KK102071/+;pdfGal4/+ | 16 | 94 | 23.50 ± 0.05 |
| w1118;+/KK102397 (Ptp36E) | 16 | 100 | 23.63 ± 0.07 |
| w1118;timGal4/KK102397 | 14 | 100 | 24.21 ± 0.11 |
| w1118;KK102397/+;timGal4/+ | 15 | 93 | 24.12 ± 0.14 |
| w1118;KK102397/+;pdfGal4/+ | 15 | 80 | 23.54 ± 0.09 |
| w1118;+/KK102474 (CG31469) | 15 | 100 | 23.73 ± 0.08 |
| w1118;timGal4/KK102474 | 16 | 100 | 24.59 ± 0.12 |
| w1118;KK102474/+;timGal4/+ | 14 | 93 | 23.73 ± 0.09 |
| w1118;KK102474/+;pdfGal4/+ | 16 | 94 | 23.77 ± 0.08 |
| w1118;+/KK103044 (Gbs-76A) | 16 | 81 | 23.38 ± 0.06 |
| w1118;timGal4/KK103044 | 16 | 75 | 23.71 ± 0.11 |
| w1118;KK103044/+;timGal4/+ | 15 | 67 | 24.85 ± 0.10 |
| w1118;KK103044/+;pdfGal4/+ | 14 | 100 | 23.93 ± 0.07 |
| w1118;+/KK103144 (Pp2B-14D) | 16 | 94 | 23.47 ± 0.06 |
| w1118;timGal4/KK103144 | 16 | 56 | 24.11 ± 0.27 |
| w1118;KK103144/+;timGal4/+ | 10 | 70 | 24.21 ± 0.32 |
| w1118;KK103144/+;pdfGal4/+ | 13 | 92 | 23.88 ± 0.13 |
| w1118;+/KK103317 (CG32568) | 16 | 100 | 23.53 ± 0.03 |
| w1118;timGal4/KK103317 | 16 | 94 | 23.83 ± 0.12 |
| w1118;KK103317/+;timGal4/+ | 12 | 100 | 23.96 ± 0.12 |
| w1118;KK103317/+;pdfGal4/+ | 16 | 100 | 23.53 ± 0.05 |
| w1118;+/KK103354 (CG7115) | 15 | 100 | 23.6 ± 0.05 |
| w1118;timGal4/KK103354 | 14 | 86 | 24.88 ± 0.2 |
| w1118;KK103354/+;timGal4/+ | 7 | 54 | 25.0 ± 0.18 |
| w1118;KK103354/+;pdfGal4/+ | 12 | 0 | AR |
| w1118;+/KK103357 (Cep97) | 15 | 100 | 23.57 ± 0.08 |
| w1118;timGal4/KK103357 | 12 | 25 | 23.83 ± 0.27 |
| w1118;KK103357/+;timGal4/+ | 13 | 8 | 25.0 |
| w1118;KK103357/+;pdfGal4/+ | 10 | 0 | AR |
| w1118;+/KK103627 (cdc14) | 16 | 100 | 23.34 ± 0.06 |
| w1118;timGal4/KK103627 | 15 | 73 | 24.05 ± 0.22 |
| w1118;KK103627/+;timGal4/+ | 15 | 93 | 25.32 ± 0.14 |
| w1118;KK103627/+;pdfGal4/+ | 13 | 8 | 26.5 |
| w1118;+/KK103740 (Ptpmeg) | 16 | 94 | 23.40 ± 0.05 |
| w1118;timGal4/KK103740 | 16 | 88 | 23.54 ± 0.03 |
| w1118;KK103740/+;timGal4/+ | 15 | 87 | 23.77 ± 0.14 |
| w1118;KK103740/+;pdfGal4/+ | 16 | 100 | 23.5 |
| w1118;+/KK104081 (CG32812) | 11 | 100 | 23.5 |
| w1118;timGal4/KK104081 | 16 | 100 | 23.63 ± 0.07 |
| w1118;KK104081/+;timGal4/+ | 16 | 88 | 23.61 ± 0.08 |
| w1118;KK104081/+;pdfGal4/+ | 16 | 94 | 23.73 ± 0.09 |
| w1118;+/KK104167 (tws) | 13 | 54 | 23.14 ± 0.09 |
| w1118;timGal4/KK104167 | 15 | 87 | 23.85 ± 0.14 |
| w1118;KK104167/+;timGal4/+ | 12 | 75 | 23.89 ± 0.20 |
| w1118;KK104167/+;pdfGal4/+ | 16 | 94 | 23.80 ± 0.13 |
| w1118;+/KK104211 (PpD6) | 15 | 93 | 23.42 ± 0.04 |
| w1118;timGal4/KK104211 | 14 | 29 | 24.88 ± 0.52 |
| w1118;KK104211/+;timGal4/+ | 12 | 58 | 25.14 ± 0.37 |
| w1118;KK104211/+;pdfGal4/+ | 12 | 8 | 25.5 |
| w1118;+/KK104374 (Mkp) | 14 | 86 | 23.5 |
| w1118;timGal4/KK104374 | 16 | 88 | 23.46 ± 0.04 |
| w1118;KK104374/+;timGal4/+ | 14 | 93 | 24.00 ± 0.17 |
| w1118;KK104374/+;pdfGal4/+ | 15 | 100 | 24.03 ± 0.06 |
| w1118;+/KK104427 (Ptpmeg2) | 16 | 94 | 23.57 ± 0.12 |
| w1118;timGal4/KK104427 | 13 | 0 | AR |
| w1118;KK104427/+;timGal4/+ | 13 | 54 | 25.00 ± 0.68 |
| w1118;KK104427/+;pdfGal4/+ | 10 | 10 | 23.5 |
| w1118;+/KK104452 (PpD5) | 16 | 100 | 23.50 ± 0.04 |
| w1118;timGal4/KK104452 | 16 | 44 | 25.29 ± 0.33 |
| w1118;KK104452/+;timGal4/+ | 15 | 67 | 24.35 ± 0.21 |
| w1118;KK104452/+;pdfGal4/+ | 13 | 15 | 23.25 ± 0.18 |
| w1118;+/KK104677 (flw) | 15 | 100 | 23.5 |
| w1118;timGal4/KK104677 | 16 | 94 | 24.5 |
| w1118;KK104677/+;timGal4/+ | 16 | 100 | 23.97 ± 0.13 |
| w1118;KK104677/+;pdfGal4/+ | 16 | 100 | 24.31 ± 0.08 |
| w1118;+/KK104729 (CG11597) | 16 | 88 | 23.29 ± 0.07 |
| w1118;timGal4/KK104729 | 13 | 69 | 23.61 ± 0.07 |
| w1118;KK104729/+;timGal4/+ | 11 | 91 | 23.70 ± 0.08 |
| w1118;KK104729/+;pdfGal4/+ | 16 | 94 | 23.80 ± 0.12 |
| w1118;+/KK104761 (Ptp69D) | 15 | 100 | 23.53 ± 0.03 |
| w1118;timGal4/KK104761 | 12 | 17 | 24.75 ± 0.18 |
| w1118;KK104761/+;timGal4/+ | 13 | 54 | 25.43 ± 0.34 |
| w1118;KK104761/+;pdfGal4/+ | 8 | 0 | AR |
| w1118;+/KK104774 (PTPMT1) | 15 | 100 | 23.47 ± 0.03 |
| w1118;timGal4/KK104774 | 15 | 93 | 24.18 ± 0.14 |
| w1118;KK104774/+;timGal4/+ | 14 | 86 | 24.63 ± 0.24 |
| w1118;KK104774/+;pdfGal4/+ | 8 | 88 | 23.36 ± 0.09 |
| w1118;+/KK104785 (Dd) | 16 | 100 | 23.03 ± 0.03 |
| w1118;timGal4/KK104785 | 16 | 100 | 23.94 ± 0.13 |
| w1118;KK104785/+;timGal4/+ | 16 | 100 | 23.97 ± 0.11 |
| w1118;KK104785/+;pdfGal4/+ | 16 | 94 | 23.70 ± 0.15 |
| w1118;+/KK104860 (mop) | 16 | 100 | 23.5 |
| w1118;timGal4/KK104860 | 13 | 92 | 24.25 ± 0.14 |
| w1118;KK104860/+;timGal4/+ | 15 | 80 | 24.83 ± 0.31 |
| w1118;KK104860/+;pdfGal4/+ | 16 | 81 | 23.62 ± 0.11 |
| w1118;+/KK104884 (MKP-4) | 16 | 90 | 23.37 ± 0.04 |
| w1118;timGal4/KK104884 | 15 | 33 | 24.40 ± 0.40 |
| w1118;KK104884/+;timGal4/+ | 12 | 67 | 25.44 ± 0.18 |
| w1118;KK104884/+;pdfGal4/+ | 11 | 27 | 24.00 ± 0.41 |
| w1118;+/KK105122 (CG13197) | 16 | 88 | 23.46 ± 0.03 |
| w1118;timGal4/KK105122 | 16 | 94 | 23.83 ± 0.12 |
| w1118;KK105122/+;timGal4/+ | 16 | 100 | 23.69 ± 0.12 |
| w1118;KK105122/+;pdfGal4/+ | 15 | 100 | 24.07 ± 0.16 |
| w1118;+/KK105249 (Pp2C) | 16 | 100 | 23.33 ± 0.04 |
| w1118;timGal4/KK105249 | 16 | 31 | 24.20 ± 0.30 |
| w1118;KK105249/+;timGal4/+ | 13 | 62 | 25.56 ± 0.20 |
| w1118;KK105249/+;pdfGal4/+ | 16 | 100 | 23.78 ± 0.08 |
| w1118;+/KK105399 (PPP4R2r) | 13 | 100 | 23.65 ± 0.05 |
| w1118;timGal4/KK105399 | 12 | 25 | 23.17 ± 0.17 |
| w1118;KK105399/+;timGal4/+ | 7 | 0 | AR |
| w1118;KK105399/+;pdfGal4/+ | 9 | 78 | 23.00 ± 0.25 |
| w1118;+/KK105483 (alph) | 16 | 100 | 23.5 |
| w1118;timGal4/KK105483 | 16 | 88 | 23.89 ± 0.10 |
| w1118;KK105483/+;timGal4/+ | 16 | 94 | 23.73 ± 0.13 |
| w1118;KK105483/+;pdfGal4/+ | 13 | 100 | 24.50 ± 0.13 |
| w1118;+/KK105484 (CG15528) | 13 | 77 | 23.25 ± 0.08 |
| w1118;timGal4/KK105484 | 16 | 50 | 23.69 ± 0.26 |
| w1118;KK105484/+;timGal4/+ | 14 | 29 | 23.63 ± 0.27 |
| w1118;KK105484/+;pdfGal4/+ | 15 | 100 | 23.90 ± 0.05 |
| w1118;+/KK105525 (Pp1α-96A) | 16 | 100 | 23.57 ± 0.05 |
| w1118;timGal4/KK105525 | 10 | 50 | 25.20 ± 0.34 |
| w1118;KK105525/+;timGal4/+ | 13 | 85 | 25.50 ± 0.13 |
| w1118;KK105525/+;pdfGal4/+ | 16 | 94 | 24.73 ± 0.12 |
| w1118;+/KK105565 (I-t) | 14 | 100 | 23.5 |
| w1118;timGal4/KK105565 | 15 | 53 | 23.63 ± 0.08 |
| w1118;KK105565/+;timGal4/+ | 16 | 56 | 23.56 ± 0.05 |
| w1118;KK105565/+;pdfGal4/+ | 11 | 55 | 23.47 ± 0.06 |
| w1118;+/KK105568 (CG6036) | 16 | 100 | 23.5 |
| w1118;timGal4/KK105568 | 16 | 56 | 24.28 ± 0.12 |
| w1118;KK105568/+;timGal4/+ | 14 | 71 | 23.90 ± 0.19 |
| w1118;KK105568/+;pdfGal4/+ | 12 | 92 | 23.68 ± 0.10 |
| w1118;+/KK105674 (CG5026) | 16 | 100 | 23.47 ± 0.03 |
| w1118;timGal4/KK105674 | 16 | 100 | 24.19 ± 0.12 |
| w1118;KK105674/+;timGal4/+ | 16 | 100 | 23.97 ± 0.13 |
| w1118;KK105674/+;pdfGal4/+ | 16 | 100 | 23.5 |
| w1118;+/KK106098 (CG7378) | 16 | 100 | 23.53 ± 0.03 |
| w1118;timGal4/KK106098 | 16 | 69 | 24.14 ± 0.27 |
| w1118;KK106098/+;timGal4/+ | 16 | 94 | 25.07 ± 0.15 |
| w1118;KK106098/+;pdfGal4/+ | 13 | 54 | 24.00 ± 0.49 |
| w1118;+/KK106180 (CG10417) | 16 | 94 | 23.50 ± 0.05 |
| w1118;timGal4/KK106180 | 14 | 14 | 24.75 ± 0.18 |
| w1118;KK106180/+;timGal4/+ | 15 | 73 | 24.64 ± 0.27 |
| w1118;KK106180/+;pdfGal4/+ | 16 | 13 | 23.25 ± 0.18 |
| w1118;+/KK106253 (Fcp1) | 16 | 100 | 23.53 ± 0.03 |
| w1118;timGal4/KK106253 | 11 | 100 | 24.36 ± 0.15 |
| w1118;KK106253/+;timGal4/+ | 4 | 100 | 23.75 ± 0.22 |
| w1118;KK106253/+;pdfGal4/+ | 16 | 100 | 23.47 ± 0.03 |
| w1118;+/KK107057 (PP2A-B’) | 31 | 87 | 23.48 ± 0.06 |
| w1118;timGal4/KK107057 | 16 | 50 | 22.25 ± 0.34 |
| w1118;KK107057/+;timGal4/+ | 12 | 42 | 22.30 ± 0.34 |
| w1118;KK107057/+;pdfGal4/+ | 16 | 81 | 23.65 ± 0.14 |
| w1118;+/KK107386 (PpD3) | 8 | 100 | 23.5 |
| w1118;timGal4/KK107386 | 12 | 100 | 24.04 ± 0.12 |
| w1118;KK107386/+;timGal4/+ | 11 | 100 | 23.68 ± 0.16 |
| w1118;KK107386/+;pdfGal4/+ | 15 | 100 | 23.57 ± 0.04 |
| w1118;+/KK107621 (CG4733) | 16 | 100 | 23.56 ± 0.04 |
| w1118;timGal4/KK107621 | 16 | 81 | 23.65 ± 0.09 |
| w1118;KK107621/+;timGal4/+ | 16 | 100 | 23.66 ± 0.07 |
| w1118;KK107621/+;pdfGal4/+ | 15 | 100 | 23.63 ± 0.10 |
| w1118;+/KK107770 (Pp1-13C) | 15 | 100 | 23.43 ± 0.04 |
| w1118;timGal4/KK107770 | 16 | 100 | 23.44 ± 0.08 |
| w1118;KK107770/+;timGal4/+ | 16 | 94 | 23.63 ± 0.07 |
| w1118;KK107770/+;pdfGal4/+ | 15 | 93 | 23.43 ± 0.05 |
| w1118;+/KK107996 (Lar) | 16 | 100 | 23.53 ± 0.03 |
| w1118;timGal4/KK107996 | 12 | 25 | 24.17 ± 0.27 |
| w1118;KK107996/+;timGal4/+ | 16 | 0 | AR |
| w1118;KK107996/+;pdfGal4/+ | 13 | 0 | AR |
| w1118;+/KK107998 (ssh) | 16 | 100 | 23.5 |
| w1118;timGal4/KK107998 | 15 | 93 | 24.07 ± 0.10 |
| w1118;KK107998/+;timGal4/+ | 15 | 100 | 24.30 ± 0.15 |
| w1118;KK107998/+;pdfGal4/+ | 16 | 100 | 23.44 ± 0.04 |
| w1118;+/KK108071 (eya) | 16 | 100 | 23.5 |
| w1118;timGal4/KK108071 | 16 | 88 | 24.11 ± 0.13 |
| w1118;KK108071/+;timGal4/+ | 16 | 100 | 23.66 ± 0.09 |
| w1118;KK108071/+;pdfGal4/+ | 16 | 100 | 23.5 |
| w1118;+/KK108352 (csw) | 15 | 100 | 23.60 ± 0.05 |
| w1118;timGal4/KK108352 | 16 | 100 | 24.41 ± 0.08 |
| w1118;KK108352/+;timGal4/+ | 16 | 94 | 24.23 ± 0.09 |
| w1118;KK108352/+;pdfGal4/+ | 15 | 87 | 23.62 ± 0.08 |
| w1118;+/KK108505 (Ptp99A) | 16 | 100 | 23.5 |
| w1118;timGal4/KK108505 | 13 | 92 | 23.71 ± 0.11 |
| w1118;KK108505/+;timGal4/+ | 16 | 100 | 23.56 ± 0.04 |
| w1118;KK108505/+;pdfGal4/+ | 16 | 100 | 23.75 ± 0.09 |
| w1118;+/KK108744 (CG10089) | 15 | 87 | 23.38 ± 0.10 |
| w1118;timGal4/KK108744 | 14 | 86 | 24.00 ± 0.12 |
| w1118;KK108744/+;timGal4/+ | 15 | 47 | 24.29 ± 0.14 |
| w1118;KK108744/+;pdfGal4/+ | 16 | 81 | 23.73 ± 0.14 |
| w1118;+/KK108802 (CG8509) | 14 | 100 | 23.54 ± 0.03 |
| w1118;timGal4/KK108802 | 14 | 93 | 24.00 ± 0.09 |
| w1118;KK108802/+;timGal4/+ | 16 | 81 | 24.15 ± 0.14 |
| w1118;KK108802/+;pdfGal4/+ | 15 | 93 | 23.79 ± 0.07 |
| w1118;+/KK108859 (NIPP1) | 15 | 80 | 23.46 ± 0.09 |
| w1118;timGal4/KK108859 | 16 | 75 | 23.86 ± 0.13 |
| w1118;KK108859/+;timGal4/+ | 12 | 83 | 24.71 ± 0.23 |
| w1118;KK108859/+;pdfGal4/+ | 15 | 100 | 23.80 ± 0.09 |
| w1118;+/KK108888 (Ptp61F) | 16 | 100 | 23.72 ± 0.08 |
| w1118;timGal4/KK108888 | 16 | 94 | 23.70 ± 0.14 |
| w1118;KK108888/+;timGal4/+ | 16 | 94 | 23.67 ± 0.09 |
| w1118;KK108888/+;pdfGal4/+ | 16 | 94 | 23.70 ± 0.10 |
| w1118;+/KK109147 (PP1-Y2) | 16 | 100 | 23.75 ± 0.06 |
| w1118;timGal4/KK109147 | 14 | 14 | 23.5 |
| w1118;KK109147/+;timGal4/+ | 11 | 18 | 24.75 ± 0.18 |
| w1118;KK109147/+;pdfGal4/+ | 12 | 67 | 25.06 ± 0.78 |
| w1118;+/KK109622 (CG14411) | 16 | 94 | 23.53 ± 0.03 |
| w1118;timGal4/KK109622 | 16 | 100 | 24.50 ± 0.13 |
| w1118;KK109622/+;timGal4/+ | 12 | 75 | 24.06 ± 0.17 |
| w1118;KK109622/+;pdfGal4/+ | 16 | 94 | 23.60 ± 0.10 |
| w1118;+/KK109858 (CanA-14F) | 16 | 100 | 23.5 |
| w1118;timGal4/KK109858 | 11 | 45 | 24.08 ± 0.27 |
| w1118;KK109858/+;timGal4/+ | 10 | 10 | 23.5 |
| w1118;KK109858/+;pdfGal4/+ | 11 | 0 | AR |
| w1118;+/KK110167 (CG3632) | 16 | 100 | 23.5 |
| w1118;timGal4/KK110167 | 16 | 94 | 23.70 ± 0.10 |
| w1118;KK110167/+;timGal4/+ | 15 | 87 | 23.73 ± 0.21 |
| w1118;KK110167/+;pdfGal4/+ | 13 | 85 | 23.45 ± 0.04 |
| w1118;+/KK110360 (Phlpp) | 16 | 88 | 23.40 ± 0.07 |
| w1118;timGal4/KK110360 | 14 | 93 | 24.00 ± 0.08 |
| w1118;KK110360/+;timGal4/+ | 16 | 100 | 23.75 ± 0.10 |
| w1118;KK110360/+;pdfGal4/+ | 16 | 81 | 23.81 ± 0.27 |
| w1118;+/KK110443 (Ptp10D) | 16 | 100 | 23.59 ± 0.05 |
| w1118;timGal4/KK110443 | 15 | 73 | 24.05 ± 0.25 |
| w1118;KK110443/+;timGal4/+ | 13 | 69 | 25.28 ± 0.26 |
| w1118;KK110443/+;pdfGal4/+ | 14 | 71 | 23.55 ± 0.22 |
| w1118;+/KK110595 (IA-2) | 16 | 100 | 23.72 ± 0.12 |
| w1118;timGal4/KK110595 | 12 | 67 | 24.42 ± 0.08 |
| w1118;KK110595/+;timGal4/+ | 16 | 63 | 24.70 ± 0.08 |
| w1118;KK110595/+;pdfGal4/+ | 5 | 80 | 24.50 ± 0.31 |
| w1118;+/KK110786 (CG3530) | 16 | 100 | 23.5 |
| w1118;timGal4/KK110786 | 16 | 88 | 25.36 ± 0.08 |
| w1118;KK110786/+;timGal4/+ | 16 | 94 | 25.37 ± 0.11 |
| w1118;KK110786/+;pdfGal4/+ | 14 | 7 | 27.0 |
Adult males were entrained in LD for 3 d and transferred to DD for at least 7 d. Analysis of activity rhythms in DD and fly genotypes are as described in Methods and Materials. For each RNAi line tested, the gene name or CG number is listed in parenthesis after the control RNAi only genotype. N, number of animals tested; % Rhythmic, percentage of rhythmic animals; Period ± SEM, rhythm period in hours ± SEM. Genotypes that lack SEM values all fell into the same half hour period increment. Bold “% Rhythmic” values signify <50% rhythmicity, bold “Period ± SEM” values are significantly different (p ≤ 0.05) from their respective UAS-RNAi/+ control flies, and “—” indicates that flies of the given genotype did not survive the run of the assay. AR, arrhythmic; LD, 12h:12h light-dark cycle; DD, complete darkness; RNAi, RNA interference.
Based on these criteria, candidate phosphatases that mediate Drosophila circadian clock function include: mts (GD41924), Pp2A-29B (GD49671), CG6380 (KK100121), CG17746 (KK100178), Gbs-70E (KK100593), Ppm1 (KK101257), I-2 (KK101547), CG7115 (KK103354), Cep97 (KK103357), PpD6 (KK104211), Ptpmeg2 (KK104427), Ptp69D (KK104761), MKP-4 (KK104884), Pp1α-96A (KK105525), CG10417 (KK106180), Lar (KK107996), Pp1-Y2 (KK109147), CanA-14F (KK109858), and CG3530 (KK110786). Next, the efficacy and specificity of RNAi-mediated knockdown of these candidate “clock phosphatases” was validated by testing RNAi lines that target another region of the mRNA and/or other genetic reagents (i.e., transposon inserts, Gal4/UAS system driven overexpression, and existing loss-of-function mutants) for activity rhythm defects. For each candidate clock phosphatase, we provide a description of the protein, its known functions, and the results of the genetic reagents used to verify the RNAi phenotype below.
Microtubule star (mts)
MTS is the catalytic subunit of a protein phosphatase 2a (Pp2A) that dephosphorylates proteins at serine and threonine residues. It functions in many cellular process including the mitotic cell cycle, cell surface receptor signaling, and cell adhesion (Janssens and Goris 2001; Chen et al. 2007). Importantly, previous work demonstrated that MTS modulates PER nuclear localization and CLK transcriptional activity within the Drosophila circadian clock (Sathyanarayanan et al. 2004; Andreazza et al. 2015). Behavioral analysis of an additional RNAi line that targeted another region of the mRNA did not validate the initial screen phenotype, emphasizing the importance of validating RNAi phenotypes using independent genetic reagents. In this case, overexpressing mts (UAS-mts) using clock cell-specific Gal4 drivers disrupted activity rhythms, thus independently validating the RNAi screen results (Table 2).
Table 2. Activity rhythms of strains used to validate candidate clock phosphatases.
| Genotype | n | % Rhythmic | Period Mean ± SEM |
|---|---|---|---|
| w1118;+/GD35171 | 43 | 100 | 23.73 ± 0.04 |
| w1118;timGal4/GD35171 | 16 | 94 | 24.43 ± 0.08 |
| w1118;GD35171/+;timGal4/+ | 11 | 91 | 24.50 ± 0.19 |
| w1118;GD35171/+;pdfGal4/+ | 15 | 93 | 23.82 ± 0.07 |
| w1118;;UAS-mts/+ | 12 | 100 | 23.5 |
| w1118;timGal4/+;UAS-mts/+ | 8 | 0 | AR |
| w1118;;UAS-mts/pdfGal4 | 14 | 43 | 23.30 ± 0.18 |
| w1118;P{EP}Pp2A-29BEP2332/+ | 16 | 100 | 23.63 ± 0.05 |
| w1118;P{EP}Pp2A-29BEP2332/timGal4 | 15 | 100 | 23.90 ± 0.13 |
| w1118;P{RS3}Pp2A-29BCB-5426-3 | 16 | 88 | 23.57 ± 0.05 |
| w1118;PBac{WH}CG17746f05041 | 11 | 91 | 23.30 ± 0.08 |
| y1w*;P{EP}CG17746G4827 | 16 | 94 | 23.53 ± 0.06 |
| y1w1118;PBac{3HPy+}I-2C362 | 14 | 100 | 23.75 ± 0.13 |
| w*;P{UAS-I-2.HA}G/+;P{UAS-Pp1-87B.HA}1H/+ | 15 | 100 | 23.67 ± 0.11 |
| w*;P{UAS-I-2.HA}G/timGal4;P{UAS-Pp1-87B.HA}1H/+ | 15 | 87 | 24.15 ± 0.15 |
| w*;P{UAS-I-2.HA}G/+;P{UAS-Pp1-87B.HA}1H/pdfGal4 | 9 | 100 | 24.22 ± 0.11 |
| y1w67c23;P{SUPor-P}CG7115KG02655 | 12 | 75 | 23.50 ± 0.14 |
| w1118;UAS-Cep97/+ | 15 | 100 | 24.27 ± 0.14 |
| w1118;UAS-Cep97/timGal4 | 9 | 100 | 24.28 ± 0.08 |
| w1118;UAS-Cep97/+;pdfGal4/+ | 13 | 100 | 24.67 ± 0.54 |
| UAS-Cep97/Y | 14 | 93 | 23.38 ± 0.06 |
| UAS-Cep97/Y;timGal4/+ | 15 | 60 | 23.83 ± 0.11 |
| UAS-Cep97/Y;;pdfGal4/+ | 13 | 85 | 23.59 ± 0.06 |
| w1118;;UAS-Cep97/+ | 15 | 100 | 23.5 |
| w1118;timGal4/+;UAS-Cep97/+ | 16 | 100 | 23.97 ± 0.10 |
| w1118;;UAS-Cep97/pdfGal4 | 16 | 100 | 24.31 ± 0.10 |
| y1;P{SUPor-P}tocKG08989PpD6KG08989 | 11 | 100 | 23.68 ± 0.12 |
| y1w*Mi{MIC}Ptpmeg2MI03011/Y | 15 | 100 | 23.73 ± 0.08 |
| w67c23P{lacW}Ptpmeg2G0232/Y | 16 | 63 | 24.65 ± 0.21 |
| y1w67c23P{Mae-UAS.6.11}Ptpmeg2GG01129/Y | 16 | 94 | 23.57 ± 0.07 |
| y1w67c23P{Mae-UAS.6.11}Ptpmeg2GG01129/Y;timGal4/+ | 10 | 90 | 24.22 ± 0.19 |
| y1w67c23P{Mae-UAS.6.11}Ptpmeg2GG01129/Y;;pdfGal4/+ | 15 | 100 | 23.80 ± 0.09 |
| w1118PBac{WH}Ptpmeg2f06600/Y | 13 | 100 | 23.62 ± 0.11 |
| w*;;Ptp69D1 | 8 | 88 | 26.57 ± 0.21 |
| w*;;Df(3L)8ex25 | 10 | 60 | 26.5 |
| w1118;;Ptp69D10 | 15 | 93 | 26.96 ± 0.14 |
| w1118;;Ptp69D18 | 16 | 50 | 27.19 ± 0.15 |
| w1118;;Ptp69D20 | 10 | 100 | 23.60 ± 0.06 |
| w1118;;Ptp69D21 | 16 | 100 | 23.53 ± 0.30 |
| w1118;;UAS-Ptp69D/+ | 9 | 89 | 24.19 ± 0.22 |
| w1118;;UAS-Ptp69D/pdfGal4 | 12 | 67 | 24.13 ± 0.19 |
| w1118;;timGal4/+;UAS-Ptp69D/+ | 12 | 92 | 27.82 ± 0.28 |
| w1118;;UAS-DNPtp69D/+ | 15 | 100 | 23.47 ± 0.09 |
| w1118;;UAS-DNPtp69D/pdfGal4 | 8 | 63 | 27.20 ± 0.18 |
| w1118;;timGal4/+;UAS-DNPtp69D/+ | 15 | 100 | 23.50 ± 0.05 |
| w1118;;Ptp69D1iso | 8 | 100 | 23.44 ± 0.06 |
| y1P{SUPor-P}MKP-4KG03420 | 13 | 100 | 24.23 ± 0.12 |
| w1118;;P{GSV6}Pp1α-96AGS11179/+ | 16 | 100 | 23.59 ± 0.08 |
| w1118;timGal4/+;P{GSV6}Pp1α-96AGS11179/+ | 16 | 25 | 24.13 ± 0.21 |
| w1118;;P{GSV6}Pp1α-96AGS11179/pdfGal4 | 9 | 67 | 23.67 ± 0.15 |
| w1118;;Pp1α-96A2/+ | 16 | 94 | 23.77 ± 0.12 |
| w1118;;UAS-Pp1α-96A.HA/+ | 16 | 100 | 23.38 ± 0.05 |
| w1118;timGal4/+;UAS-Pp1α-96A.HA/+ | 16 | 94 | 23.87 ± 0.11 |
| w1118;;UAS-Pp1α-96A.HA/pdfGal4 | 16 | 94 | 23.33 ± 0.08 |
| w1118;;Pp1α-96A-CRISPRmutant-1/+ | 17 | 82 | 23.52 ± 0.07 |
| w1118;;Pp1α-96A-CRISPRmutant-2/+ | 13 | 92 | 23.58 ± 0.05 |
| w1118;;Pp1α-96A-CRISPRmutant-3/+ | 9 | 100 | 23.44 ± 0.05 |
| w1118;UAS-CG10417/+ | 16 | 100 | 23.44 ± 0.04 |
| w1118;UAS-CG10417/timGal4 | 14 | 100 | 24.96 ± 0.09 |
| w1118;UAS-CG10417/+;pdfGal4/+ | 16 | 100 | 24.97 ± 0.03 |
| w*;Lar13.2/+ | 14 | 93 | 23.54 ± 0.04 |
| Df(2L)TW84,l(2)74i1,amosTftLarTW84/+ | 14 | 86 | 23.71 ± 0.11 |
| Df(2L)E55,rdo1hook1LarE55pr1/+ | 16 | 88 | 24.32 ± 0.08 |
| w1118;;UAS-Lar/+ | 14 | 100 | 23.57 ± 0.04 |
| w1118;timGal4/+;UAS-Lar/+ | 15 | 93 | 24.06 ± 0.07 |
| w1118;;UAS-Lar/pdfGal4 | 16 | 75 | 24.17 ± 0.07 |
| w1118;LarDf(2L)E55/Lar13.2;+ | 14 | 0 | AR |
| w1118;UAS-CanA-14Fmyc/+ | 16 | 75 | 24.50 ± 0.20 |
| w1118;UAS-CanA-14Fmyc/timGal4 | 16 | 63 | 23.60 ± 0.10 |
| w1118;UAS-CanA-14Fmyc/+;pdfGal4/+ | 15 | 100 | 24.03 ± 0.10 |
| w1118;;UAS-CanA-14Fact-myc/+ | 9 | 100 | 23.94 ± 0.11 |
| w1118;timGal4/+;UAS-CanA-14Fact-myc/+ | 15 | 0 | AR |
| w1118;;UAS-CanA-14Fact-myc/pdfGal4 | 16 | 94 | 25.03 ± 0.15 |
| CanA-14F-KO/Y | 15 | 13 | 25.0 |
| CanA-14F-KOiso/Y | 16 | 94 | 23.67 ± 0.08 |
| w1118;GD26216/+ | 12 | 100 | 23.63 ± 0.13 |
| w1118;GD26216/timGal4 | 15 | 100 | 24.90 ± 0.13 |
| w1118;GD26216/+;timGal4/+ | 13 | 85 | 23.68 ± 0.12 |
| w1118;GD26216/+;pdfGal4/+ | 15 | 100 | 24.30 ± 0.18 |
Adult males were entrained in LD for 3 d and transferred to DD for at least 7 d. Analysis of activity rhythms in DD and fly genotypes are as described in Methods and Materials. n, number of animals tested; % Rhythmic, percentage of rhythmic animals; Period ± SEM, rhythm period in hours ± SEM. Bold “% Rhythmic” values signify < 50% rhythmicity and bold “Period ± SEM” values are significantly different (p ≤ 0.05) from their respective UAS-RNAi/+ control flies. AR, arrhythmic; LD, 1h2:12h light-dark cycle; DD, complete darkness; RNAi, RNA interference.
Pp2A-29B
Pp2A-29B is a protein phosphatase type 2A regulatory subunit. It functions in many cellular processes including chromosome segregation, centriole assembly, and phagocytosis (Stroschein-Stevenson et al. 2006; Dobbelaere et al. 2008). We tested additional P element transposon inserts to independently validate the RNAi phenotype, but activity rhythms were not altered (Table 2).
CG6380
CG6380 is related to Protein phosphatase inhibitor 2, IPP-2. Based on association of InterPro records with GO terms, it functions to regulate signal transduction and phosphoprotein phosphatase pathways. Although the phenotype of this RNAi knockdown was strongly arrhythmic, no other genetic reagents were available to confirm the RNAi phenotype.
CG17746
CG17746 is a member of the protein phosphatase 2C family that has cation binding domains and dephosphorylates proteins at serine and threonine residues. We tested additional P element transposon inserts for activity rhythms; however, these reagents did not validate the RNAi phenotype (Table 2).
Gbs-70E
Gbs-70E is a protein phosphatase 1 regulatory subunit with a carbohydrate binding type-21 (CBM21) domain. It functions in regulation of glycogen metabolic process (Kerekes et al. 2014). However, there are no additional genetic reagents available for this gene to validate the arrhythmic RNAi phenotype.
Ppm1
PPM1 is a protein phosphatase, Mg+2/Mn+2-dependent (PPM type), member of the protein phosphatase 2C family that dephosphorylates proteins at serine and threonine residues. There are no known cellular functions described for this phosphatase, and no additional genetic reagents available to validate the RNAi phenotype.
I-2
I-2 is a protein phosphatase inhibitor with protein phosphatase 1 binding activity (Sami et al. 2011). Behavioral analysis of P element transposon insert and overexpression (UAS-I-2) driven with clock cell-specific Gal4 drivers did not reproduce a defect in activity rhythms consistent with the RNAi knockdown (Table 2).
CG7115
CG7115 is a cation binding, PPM-type phosphatase, part of the protein phosphatase 2C family that dephosphorylates proteins at serine and threonine residues. It functions in many cellular processes including cell adhesion and regulation of cell shape (Sopko et al. 2014). We tested the available P element insert to independently validate the long period and/or arrhythmicity associated with RNAi, but did not observe any alteration in activity rhythms (Table 2).
Cep97
Cep97 is a protein phosphatase type 1 regulator with a characteristic leucine-rich repeat domain. It is known to function in centriole replication (Dobbelaere et al. 2008). Behavioral analysis of Cep97 overexpression using clock cell-specific Gal4 drivers did not alter activity rhythms (Table 2).
PpD6
PpD6 is a protein phosphatase that dephosphorylates proteins at serine and threonine residues. There are no known cellular functions described for this phosphatase. We tested the only available P element transposon insert for this gene, but activity rhythms in this strain were not altered (Table 2).
Ptpmeg2
Ptpmeg2, also known as lethal-1-G0232, is a nonmembrane spanning protein tyrosine phosphatase. It functions in many cellular processes including phagocytosis, neurogenesis, and cell migration (Stroschein-Stevenson et al. 2006; Chen et al. 2012). We tested the available Ptpmeg2 P element transposon insert lines and clock cell-specific Ptpmeg2 overexpression flies, but none of these genetic reagents altered activity rhythms (Table 2).
Ptp69D
Ptp69D is a protein tyrosine phosphatase with characteristic Fibronectin type III, Immunoglobulin subtype, and tyrosine-specific protein phosphatase domains. It is a transmembrane RPTP that dephosphorylates protein’s tyrosine residues. It functions in many cellular processes including dendrite morphogenesis, axon guidance, and fasciculation-defasciculation of neuron axons (Desai et al. 1996; Desai and Purdy 2003). Interestingly, when we tested additional Ptp69D reagents including loss-of-function mutants and a dominant negative UAS strain, many showed an even longer period phenotype compared to the RNAi while some showed no phenotype (Table 2). When Ptp69D mutants that showed a long period were isogenized to the wild-type (w1118) reference strain or paired with a wild-type X chromosome (data not shown), the long period phenotype was lost (Table 2). Upon further analysis (see Methods and Materials), we confirmed that the long period (∼26.5 hr) phenotype was due to perSLIH, a naturally occurring per mutant (Hamblen et al. 1998).
MKP-4
MKP-4 is a dual specificity protein phosphatase that dephosphorylates proteins at tyrosine, serine, and threonine residues. Its known cellular function is in negative regulation of JUN kinase activity (Sun et al. 2008). We tested an additional P element transposon insert to independently validate the RNAi phenotype but activity rhythms were not altered (Table 2).
Pp1α-96A
Pp1α-96A is a protein phosphatase, part of the PP1 subfamily, which dephosphorylates proteins at serine and threonine residues. It functions in many cellular processes including positive regulation of the canonical Wnt signaling pathway and innate immune response (Schertel et al. 2013). Importantly, the PP1 subfamily is proposed to regulate clock function in Drosophila by maintaining rhythms in PER-TIM abundance (Fang et al. 2007). None of the available Pp1α-96A P element inserts or clock cell-specific Pp1α-96A overexpression altered activity rhythms (Table 2). Given the involvement of PP1 in Drosophila circadian clocks, we wanted to test loss-of-function mutants. Therefore, we used CRISPR technology to generate three Pp1α-96A deletion mutants (See Methods and Materials). However, none of these mutants were homozygous viable as adults, and heterozygotes did not display altered activity rhythms (Table 2).
CG10417
CG10417 is a PPM-type phosphatase, a member of the protein phosphatase 2C family that dephosphorylates proteins at the serine and threonine residues (Sopko et al. 2014). No other genetic reagents were available for this gene. However, given its association with the PP2 family known to be involved in Drosophila circadian clock function (Sathyanarayanan et al. 2004), we generated a UAS-CG10417 strain to overexpress this phosphatase in clock cells. Interestingly, CG10417 overexpression in clock cells resulted in a long period phenotype (Table 2). This phenotype is similar to that of the RNAi knockdown, demonstrating that increasing or decreasing the dephosphorylation of CG10417 targets slows the pace of the clock. This is not unprecedented, since RNAi knockdown and overexpression of the PP2A subunit WDB also leads to long period rhythms (Sathyanarayanan et al. 2004; Andreazza et al. 2015). Since CG10417 RNAi knockdown and overexpression both lengthened period, we investigated whether either manipulation altered CLK, PER, or TIM protein levels or phosphorylation, but no obvious change in either parameter was detected (data not shown).
Lar
LAR is a transmembrane RPTP bearing Fibronectin type III, Immunoglobulin-like, and tyrosine-specific protein phosphatase domains. It is a transmembrane receptor protein tyrosine phosphatase that dephosphorylates proteins tyrosine residues (Streuli et al. 1989). It functions in many cellular processes including cell adhesion, axon guidance, and regulation of nervous system development (Krueger et al. 1996, 2003; Kiger et al. 2003). Although multiple loss-of-function mutants were available for this phosphatase, none were homozygous viable. However, one heterozygous combination of Lar loss-of-function alleles survived and phenocopied the arrhythmicity seen in Lar RNAi knockdown flies (Table 2). Further analysis showed that Lar is required for the development of circadian pacemaker neuron processes required for activity rhythms during constant darkness but not light:dark cycles (Agrawal and Hardin 2016).
Pp1-Y2
Pp1-Y2 is a protein phosphatase that dephosphorylates proteins at the serine and threonine residues. The RNAi shows a strong arrhythmic and/or long period phenotype, but no additional genetic reagents were available to validate the RNAi phenotype.
CanA-14F
CanA-14F is a protein phosphatase that dephosphorylates proteins at serine and threonine residues. It functions in many cellular processes including positive regulation of nuclear factor of activated T cells (NFAT) protein import into nucleus (Shibasaki et al. 1996) and sleep (Nakai et al. 2011). A CanA-14F knockout (KO) and a constitutively active CanA-14F form (expressed via Gal4/UAS) were generated previously (Nakai et al. 2011), and showed strong arrhythmic and long period activity phenotypes, respectively (Table 2). However, the arrhythmicity associated with CanA-14F KOs was lost when this allele was isogenized to a wild-type (w1118) reference strain (Table 2).
CG3530
CG3530 is a protein phosphatase that dephosphorylates proteins at the tyrosine residues. Its known cellular function is in the mitotic cell cycle (Chen et al. 2007). Behavioral analysis of an additional RNAi line that targeted another region of the mRNA did not validate the initial screen phenotype (Table 2).
Discussion
An in vivo screen of 86 RNAi lines, representing the majority of annotated Drosophila phosphatases/regulators, for altered activity rhythms was carried out. The screen identified a total of 19 candidate genes (Table 1) that altered clock function upon RNAi knockdown in Drosophila clock cells. Further genetic validation of one candidate showed that the RPTP Lar is required for the development of axonal projections from circadian pacemaker neurons that support rhythmic activity in constant darkness but not during light:dark cycles (Agrawal and Hardin 2016).
As expected, a majority of these candidates were not validated upon further analysis of independent genetic reagents (Table 2). However, these reagents consisted of additional P element inserts, where the P element insertion site may not interfere with gene function, or strains that could be used for overexpression, which also may not impact the function of a protein that is already at saturating levels. Therefore, a lack of validation with P element inserts and overexpression for these candidate clock phosphatases does not eliminate them from the list of viable candidates. However, for two candidate phosphatases, Ptp69D and CanA14F, loss-of-function mutants upon isogenization did not alter activity rhythms (Table 2), therefore these can be eliminated from the list of viable candidates.
Previous studies show that PP1 and PP2a both function within the Drosophila clock (Sathyanarayanan et al. 2004; Fang et al. 2007; Andreazza et al. 2015). PP1 is comprised of a catalytic subunit that engages with one of dozens of regulatory subunits to select substrates and control enzymatic activity (Peti et al. 2013). PP1 function in the Drosophila clock was assessed previously by overexpressing the nuclear inhibitor of PP1 (NIPP1), which reduced TIM levels and lengthened circadian period, indicating that PP1 dephosphorylates and stabilizes TIM to maintain circadian period (Fang et al. 2007). Since these circadian phenotypes were produced by generically inhibiting PP1, the clock-relevant catalytic and regulatory subunits involved were not identified. Five PP1 catalytic subunit genes (flw, Pp1-13C, Pp1-87B, Pp1α-96A, and Pp1-Y2) and eight PP1 regulator genes (sds22, NIPP1, I-t, I-2, TbCMF46, Gbs-70A, Gbs-70E, and Cep97) were tested in our screen, and Pp1α-96A, Pp1-Y2, Gbs-70E, I-2, and Cep97 showed aberrant circadian phenotypes (Table 1). Further analysis of Pp1α-96A showed that none of the available Pp1α-96A P element inserts or overexpression of Pp1α-96A in clock cells altered activity rhythms (Table 2). Thus, we used the CRISPR/Cas9 system to generate three Pp1α-96A deletion mutants expected to disrupt Pp1α-96A protein expression and/or function (see Methods and Materials; Figure 1). However, none of these Pp1α-96A deletions were homozygous viable as adults, and heterozygotes did not display altered activity rhythms (Table 2). Further characterization of Pp1α-96A function in the clock will benefit from targeted loss of Pp1α-96A in clock cells. The Pp1-Y2 catalytic subunit and the Gbs-70E and I-2 regulators could not be tested further due to lack of genetic reagents and Cep97 overexpression did not alter circadian rhythms (Table 2), but each of these genes remain as viable candidate clock protein phosphatases until additional loss-of-function reagents are available to test. Although inhibiting PP1 via NIPP1 overexpression lengthened circadian period (Sathyanarayanan et al. 2004), RNAi knockdown of NIPP1 did not alter activity rhythms (Table 1). These results suggest that NIPP1 RNAi is either ineffective, NIPP1 is not involved in suppressing PP1 activity in clock cells, or increased PP1 activity does not disrupt the circadian clock.
The PP2a holoenzyme contains a structural subunit Pp2A-29B, a catalytic subunit MTS, and regulatory subunits TWS, WDB, PP2a-B’, CG4733, and Connector of kinase to AP-1 (CKA) (Andreazza et al. 2015). Previous work shows that mts, tws, and wdb overexpression, hypomorphic mutants, and/or RNAi knockdown, alter the levels and localization of PER and disrupt activity rhythms (Sathyanarayanan et al. 2004; Andreazza et al. 2015), whereas a hypomorphic cka mutant and cka RNAi knockdown reduces CLK activity and lengthens period (Andreazza et al. 2015). We tested RNAi knockdowns of all PP2a components except cka, but only mts and Pp2A-29B produced circadian phenotypes (Table 1). Our inability to generate circadian phenotypes for tws and wdb may be due to inefficient RNAi knockdown, since expressing a different wdb RNAi along with Dicer2 (to enhance RNAi potency) produced a long period phenotype (Andreazza et al. 2015). We did not test cka because it is not annotated as a PP2a subunit.
Genetic reagents for effecting a loss- or gain-of-function were not available for four additional candidate phosphatases, which can be characterized further when such reagents are available. For example, P element inserts are now available for Gbs-70E and CG3530. Another method that can be used to follow up on these candidates is to generate mutants using CRISPR technology. Since loss of most phosphatases is lethal, it is likely that generating conditional null mutants via CRISPR will provide the best opportunity to assess loss-of-function phenotypes in adults (Gratz et al. 2014).
Overall, we identified 19 protein phosphatases that may function within the Drosophila circadian clock (Table 3). Lar and mts functions have now been characterized, and they are shown to be important for dephosphorylation events that regulate fly clock development or function (Andreazza et al. 2015; Agrawal and Hardin 2016). Ptp69D and CanA-14F loss-of-function mutants do not alter activity rhythms upon isogenization, thus, identification of these genes may have been due to off-target effects of RNAi. No loss-of-function mutants are currently available for the remaining 15 candidates, and four of these candidates could not be validated due to the lack of independent genetic reagents (Table 3). We analyzed previous mRNA expression and CLK binding data that may further support a possible role for these candidates in the clock (Chintapalli et al. 2007; Celniker et al. 2009; Kula-Eversole et al. 2010; Abruzzi et al. 2011). Of the remaining 15 candidates, one is a rhythmic and two are nonrhythmic CLK binding targets, three are enriched in small LNvs, five are cycling in large LNvs, and five are highly or very highly expressed in pacemaker neuron-containing tissues (i.e., brain and head) that could account for the altered activity rhythms due to candidate gene RNAi knockdown (Table 3). The other nine candidates were not detected as highly or very highly expressed transcripts in brains and heads, but moderate levels of transcripts were found in heads or brains for five of these candidates, leaving four candidates with low or no expression in the head (Table 3). One candidate (i.e., Ppm1) that was detected in lLNv pacemaker neurons showed low or no expression in the head (Table 3), consistent with there being only eight lLNvs per head (Helfrich-Forster 2014). The strong behavioral phenotypes displayed by RNAi knockdowns of these 15 candidates suggest they are viable candidate clock phosphatases, a possibility that is further supported by data showing that nine of these candidates are either CLK binding targets or produce cycling mRNAs in clock neurons (Table 3). Additional characterization of the remaining candidates may reveal novel features of the circadian timekeeping mechanism that are conserved in all animals including humans.
Table 3. Summary of results for candidate circadian phosphatases.
| Candidate | RNAi Phenotype | Genetic Reagents Tested | Validation | Spatial Expressiona | Clock-Related Expression |
|---|---|---|---|---|---|
| mtsb | Long | Additional RNAi, overexpression | Yes | Brain, eye, tubule, carcass, ovary, heart, spermatheca, gut, fat body, head | CLK target and cyclingc |
| Pp2A-29Bb | Long | P element inserts | No | Head, brain, tubule, ovary, testis, fat body, gut, salivary gland | CLK target and noncyclingc |
| IPP-2 | AR | No reagents | — | Testis | |
| CG17746 | AR | P element inserts | No | Tubule, ovary | CLK target and noncyclingc; cycling mRNA in large PDF neuronsd |
| Gbs-70E | Long | No reagents | — | Head, carcass, ovary, heart, fat body, eye, crop, salivary gland, spermatheca | CLK target and cyclingc; cycling mRNA in large PDF neuronsd |
| Ppm1 | AR | No reagents | — | Testis | Cycling mRNA in large PDF neuronsd |
| I-2 | AR | P element insert, overexpression | No | Head, tubule, carcass, ovary, testis, spermatheca, ganglion | |
| CG7115 | Long | P element insert | No | Carcass, ovary | mRNA enriched in s-LNvsd |
| Cep97 | AR | Overexpression strains | No | Testis | |
| PpD6 | Long | P element insert | No | — | |
| Ptpmeg2 | Long | P element inserts, overexpression | No | Head | Cycling mRNA in large PDF neuronsd |
| Ptp69D | Long | Loss-of-function mutants, Dominant negative strain | Noe | Ovary | mRNA enriched in s-LNvs; cycling mRNA in small and large PDF neuronsd |
| MKP-4 | Long | P element insert | No | — | Cycling mRNA in large PDF neuronsd |
| Pp1a-96Af | Long | P element inserts, Overexpression, CRISPR heterozygous mutants | No | Head, carcass, ovary, testis, crop, fat body, gut, salivary gland, spermatheca, accessory gland | mRNA enriched in s-LNvsd |
| CG10417 | Long | Overexpression | Yes | Carcass, ovary | |
| Lar | AR | Deficiency over point mutant heterozygote | Yes | — | |
| Pp1-Y2 | Long | No reagents | — | — | |
| CanA-14Fg | AR | Knockout, expression of constitutively active form | Noh | Head, carcass, ovary | |
| CG3530 | Long | Additional RNAi | No | Brain, head, ganglion | mRNA enriched in s-LNvsd |
Bold denotes the remaining candidate clock protein phosphatases. RNAi, RNA interference; CLK, CLOCK; AR, arrhythmic; mRNA, messenger RNA; PDF, pigment dispersing factor; CRISPR, clustered regularly interspaced short palindromic repeats.
High/very high expression level in adult fly tissues based on Celniker et al. (2009) and Chintapalli et al. (2007).
Clock-related based on Sathyanarayanan et al. (2004).
Direct CLK binding target and cycling or noncycling mRNA expression based on Abruzzi et al. (2011).
Differential mRNA expression in pacemaker neurons based on Kula-Eversole et al. (2010).
All genetic reagents used to verify the RNAi phenotype that produced long period rhythms were due to perSLIH.
Clock-related based on Fang et al. (2007).
Sleep-related based on previously analyzed for circadian phenotype Nakai et al. (2011).
Knockout allele had a long period rhythm which was lost upon isogenization, but constitutively active form produces a long period rhythm upon overexpression.
Acknowledgments
This work was supported by National Institutes of Health grant NS052894.
Footnotes
Communicating editor: J. C. Dunlap
Literature Cited
- Abruzzi K. C., Rodriguez J., Menet J. S., Desrochers J., Zadina A., et al. , 2011. Drosophila CLOCK target gene characterization: implications for circadian tissue-specific gene expression. Genes Dev. 25: 2374–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal P., Hardin P. E., 2016. The Drosophila receptor protein tyrosine phosphatase LAR is required for development of circadian pacemaker neuron processes that support rhythmic activity in constant darkness but not during light/dark cycles. J. Neurosci. 36: 3860–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akten B., Jauch E., Genova G. K., Kim E. Y., Edery I., et al. , 2003. A role for CK2 in the Drosophila circadian oscillator. Nat. Neurosci. 6: 251–257. [DOI] [PubMed] [Google Scholar]
- Akten B., Tangredi M. M., Jauch E., Roberts M. A., Ng F., et al. , 2009. Ribosomal s6 kinase cooperates with casein kinase 2 to modulate the Drosophila circadian molecular oscillator. J. Neurosci. 29: 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreazza S., Bouleau S., Martin B., Lamouroux A., Ponien P., et al. , 2015. Daytime CLOCK dephosphorylation is controlled by STRIPAK complexes in Drosophila. Cell Reports 11: 1266–1279. [DOI] [PubMed] [Google Scholar]
- Celniker S. E., Dillon L. A., Gerstein M. B., Gunsalus K. C., Henikoff S., et al. , 2009. Unlocking the secrets of the genome. Nature 459: 927–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D. Y., Li M. Y., Wu S. Y., Lin Y. L., Tsai S. P., et al. , 2012. The Bro1-domain-containing protein Myopic/HDPTP coordinates with Rab4 to regulate cell adhesion and migration. J. Cell Sci. 125: 4841–4852. [DOI] [PubMed] [Google Scholar]
- Chen F., Archambault V., Kar A., Lio P., D’Avino P. P., et al. , 2007. Multiple protein phosphatases are required for mitosis in Drosophila. Curr. Biol. 17: 293–303. [DOI] [PubMed] [Google Scholar]
- Chintapalli V. R., Wang J., Dow J. A., 2007. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39: 715–720. [DOI] [PubMed] [Google Scholar]
- Chiu J. C., Vanselow J. T., Kramer A., Edery I., 2008. The phospho-occupancy of an atypical SLIMB-binding site on PERIOD that is phosphorylated by DOUBLETIME controls the pace of the clock. Genes Dev. 22: 1758–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu J. C., Ko H. W., Edery I., 2011. NEMO/NLK phosphorylates PERIOD to initiate a time-delay phosphorylation circuit that sets circadian clock speed. Cell 145: 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai C., Purdy J., 2003. The neural receptor protein tyrosine phosphatase DPTP69D is required during periods of axon outgrowth in Drosophila. Genetics 164: 575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai C. J., Gindhart J. G., Jr, Goldstein L. S., Zinn K., 1996. Receptor tyrosine phosphatases are required for motor axon guidance in the Drosophila embryo. Cell 84: 599–609. [DOI] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y., et al. , 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156. [DOI] [PubMed] [Google Scholar]
- Dobbelaere J., Josue F., Suijkerbuijk S., Baum B., Tapon N., et al. , 2008. A genome-wide RNAi screen to dissect centriole duplication and centrosome maturation in Drosophila. PLoS Biol. 6: e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Sathyanarayanan S., Sehgal A., 2007. Post-translational regulation of the Drosophila circadian clock requires protein phosphatase 1 (PP1). Genes Dev. 21: 1506–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz S. J., Ukken F. P., Rubinstein C. D., Thiede G., Donohue L. K., et al. , 2014. Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics 196: 961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz S. J., Rubinstein C. D., Harrison M. M., Wildonger J., O’Connor-Giles K. M., 2015. CRISPR-Cas9 genome editing in Drosophila. Curr. Protoc. Mol. Biol. 111: 31.2.1–31.2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblen M. J., White N. E., Emery P. T., Kaiser K., Hall J. C., 1998. Molecular and behavioral analysis of four period mutants in Drosophila melanogaster encompassing extreme short, novel long, and unorthodox arrhythmic types. Genetics 149: 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Forster C., 2014. From neurogenetic studies in the fly brain to a concept in circadian biology. J. Neurogenet. 28: 329–347. [DOI] [PubMed] [Google Scholar]
- Janssens V., Goris J., 2001. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 353: 417–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerekes E., Kokai E., Paldy F. S., Dombradi V., 2014. Functional analysis of the glycogen binding subunit CG9238/Gbs-70E of protein phosphatase 1 in Drosophila melanogaster. Insect Biochem. Mol. Biol. 49: 70–79. [DOI] [PubMed] [Google Scholar]
- Kiger A. A., Baum B., Jones S., Jones M. R., Coulson A., et al. , 2003. A functional genomic analysis of cell morphology using RNA interference. J. Biol. 2: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. Y., Edery I., 2006. Balance between DBT/CKIε kinase and protein phosphatase activities regulate phosphorylation and stability of Drosophila CLOCK protein. Proc. Natl. Acad. Sci. USA 103: 6178–6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss B., Price J. L., Saez L., Blau J., Rothenfluh A., et al. , 1998. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iε. Cell 94: 97–107. [DOI] [PubMed] [Google Scholar]
- Kloss B., Rothenfluh A., Young M. W., Saez L., 2001. Phosphorylation of period is influenced by cycling physical associations of double-time, period, and timeless in the Drosophila clock. Neuron 30: 699–706. [DOI] [PubMed] [Google Scholar]
- Krueger N. X., Van Vactor D., Wan H. I., Gelbart W. M., Goodman C. S., et al. , 1996. The transmembrane tyrosine phosphatase DLAR controls motor axon guidance in Drosophila. Cell 84: 611–622. [DOI] [PubMed] [Google Scholar]
- Krueger N. X., Reddy R. S., Johnson K., Bateman J., Kaufmann N., et al. , 2003. Functions of the ectodomain and cytoplasmic tyrosine phosphatase domains of receptor protein tyrosine phosphatase Dlar in vivo. Mol. Cell. Biol. 23: 6909–6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kula-Eversole E., Nagoshi E., Shang Y., Rodriguez J., Allada R., et al. , 2010. Surprising gene expression patterns within and between PDF-containing circadian neurons in Drosophila. Proc. Natl. Acad. Sci. USA 107: 13497–13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. M., Kilman V. L., Keegan K., Paddock B., Emery-Le M., et al. , 2002. A role for casein kinase 2alpha in the Drosophila circadian clock. Nature 420: 816–820. [DOI] [PubMed] [Google Scholar]
- Martinek S., Inonog S., Manoukian A. S., Young M. W., 2001. A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell 105: 769–779. [DOI] [PubMed] [Google Scholar]
- Nakai Y., Horiuchi J., Tsuda M., Takeo S., Akahori S., et al. , 2011. Calcineurin and its regulator sra/DSCR1 are essential for sleep in Drosophila. J. Neurosci. 31: 12759–12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peti W., Nairn A. C., Page R., 2013. Structural basis for protein phosphatase 1 regulation and specificity. FEBS J. 280: 596–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffenberger C., Lear B. C., Keegan K. P., Allada R., 2010. Sleep and circadian behavior monitoring in Drosophila, pp. 483–504 in Drosophila Neurobiology: A Laboratory Manual, edited by Zhang B., Freeman M. R., Waddell S. Cold Spring Harbor Press, Cold Spring Harbor, NY. [Google Scholar]
- Price J. L., Blau J., Rothenfluh A., Abodeely M., Kloss B., et al. , 1998. Double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell 94: 83–95. [DOI] [PubMed] [Google Scholar]
- Ren X., Sun J., Housden B. E., Hu Y., Roesel C., et al. , 2013. Optimized gene editing technology for Drosophila melanogaster using germ line-specific Cas9. Proc. Natl. Acad. Sci. USA 110: 19012–19017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami F., Smet-Nocca C., Khan M., Landrieu I., Lippens G., et al. , 2011. Molecular basis for an ancient partnership between prolyl isomerase Pin1 and phosphatase inhibitor-2. Biochemistry 50: 6567–6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayanan S., Zheng X., Xiao R., Sehgal A., 2004. Posttranslational regulation of Drosophila PERIOD protein by protein phosphatase 2A. Cell 116: 603–615. [DOI] [PubMed] [Google Scholar]
- Schertel C., Huang D., Bjorklund M., Bischof J., Yin D., et al. , 2013. Systematic screening of a Drosophila ORF library in vivo uncovers Wnt/Wg pathway components. Dev. Cell 25: 207–219. [DOI] [PubMed] [Google Scholar]
- Shibasaki F., Price E. R., Milan D., McKeon F., 1996. Role of kinases and the phosphatase calcineurin in the nuclear shuttling of transcription factor NF-AT4. Nature 382: 370–373. [DOI] [PubMed] [Google Scholar]
- Sopko R., Foos M., Vinayagam A., Zhai B., Binari R., et al. , 2014. Combining genetic perturbations and proteomics to examine kinase-phosphatase networks in Drosophila embryos. Dev. Cell 31: 114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Tsai A. Y., Saito H., 1989. A family of receptor-linked protein tyrosine phosphatases in humans and Drosophila. Proc. Natl. Acad. Sci. USA 86: 8698–8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroschein-Stevenson S. L., Foley E., O’Farrell P. H., Johnson A. D., 2006. Identification of Drosophila gene products required for phagocytosis of Candida albicans. PLoS Biol. 4: e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Yu M. C., Kong L., Zhuang Z. H., Hu J. H., et al. , 2008. Molecular identification and functional characterization of a Drosophila dual-specificity phosphatase DMKP-4 which is involved in PGN-induced activation of the JNK pathway. Cell. Signal. 20: 1329–1337. [DOI] [PubMed] [Google Scholar]
- Szabo A., Papin C., Zorn D., Ponien P., Weber F., et al. , 2013. The CK2 kinase stabilizes CLOCK and represses its activity in the Drosophila circadian oscillator. PLoS Biol. 11: e1001645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Zheng H., Houl J. H., Dauwalder B., Hardin P. E., 2006. PER-dependent rhythms in CLK phosphorylation and E-box binding regulate circadian transcription. Genes Dev. 20: 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.