Figure 2.
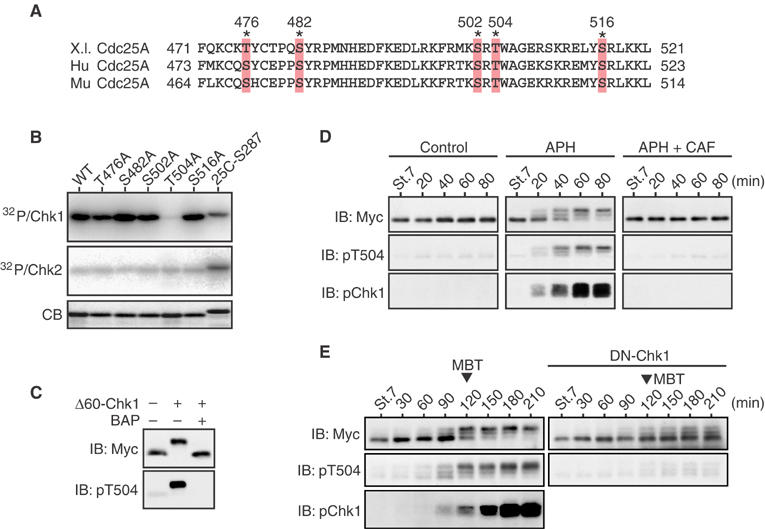
Phosphorylation of Cdc25A Thr504 by Chk1 but not Chk2. (A) Alignment of the C-terminal regions of Xenopus (X.l.), human (Hu), and mouse (Mu) Cdc25A proteins. The asterisks show conserved Ser/Thr residues. (B) GST-Cdc25A C-terminal peptide fusion proteins (each with a Ser/Thr → Ala substitution) were phosphorylated by Δ60-Chk1 or Chk2 in vitro and analyzed as in Figure 1B. (C) Extracts from the eggs expressing Myc-tagged S73A Cdc25A together with or without Δ60-Chk1 were mock-treated or treated with bacterial alkaline phosphatase (BAP) and analyzed by immunoblotting (IB) using anti-Myc or anti-phospho-Thr504 antibodies. (D) One-cell embryos were injected with 1 ng of mRNA encoding Myc-tagged S73A Cdc25A. At the early blastula stage 7, the embryos were mock-treated or treated with aphidicolin (APH) together with or without caffeine (CAF) and, at 20 min intervals, analyzed by immunoblotting using anti-Myc antibody, anti-phospho-Thr504 antibody, or anti-human Chk1 phospho-Ser345 antibody (pChk1, which can recognize well the activating phosphorylation of Xenopus Chk1; Shimuta et al, 2002). (E) One-cell embryos were coinjected with 1 ng of mRNA encoding Myc-tagged S73A Cdc25A and 15 ng of mRNA encoding either control GST (left panel) or a dominant-negative (or kinase-dead) form of Chk1 (DN-Chk1), and then analyzed as in (D).
