Abstract
Morphine is a poor inducer of μ-opioid receptor (MOR) internalization, but a potent inducer of cellular tolerance. Here we show that, in contrast to full agonists such as [D-Ala2-MePhe4-Gly-ol]enkephalin (DAMGO), morphine stimulated a selective phosphorylation of the carboxy-terminal residue 375 (Ser375). Ser375 phosphorylation was sufficient and required for morphine-induced desensitization of MOR. In the presence of full agonists, morphine revealed partial agonistic properties and potently inhibited MOR phosphorylation and internalization. Upon removal of the drug, DAMGO-desensitized receptors were rapidly dephosphorylated. In contrast, morphine-desensitized receptors remained at the plasma membrane in a Ser375-phosphorylated state for prolonged periods. Thus, morphine promotes terminal MOR desensitization by inducing a persistent modification of Ser375.
Keywords: desensitization, morphine, opioid receptor, phosphorylation, tolerance
Introduction
Morphine is a powerful pain reliever; however, the utility of morphine for the treatment of chronic pain is hindered by the development of tolerance (Nestler, 1996; Nestler and Aghajanian, 1997; Koob et al, 1998). Morphine tolerance occurs on continued use of the drug such that the amount of drug required to elicit pain relief must be increased to compensate for diminished responsiveness (Nestler, 1996; Nestler and Aghajanian, 1997; Koob et al, 1998). In many systems, decreased responsiveness to agonists has been correlated with desensitization and internalization of G protein-coupled receptors (Pitcher et al, 1998). However, morphine profoundly differs from other opioids such as [D-Ala2-MePhe4-Gly-ol]enkephalin (DAMGO) in that it activates the μ-opioid receptor (MOR, MOP) without causing its rapid internalization (Keith et al, 1996). The differential regulation of receptor endocytosis by distinct opioid agonists appears to be related to their ability to promote G protein-coupled receptor kinase (GRK)-dependent phosphorylation of MOR (Zhang et al, 1998). Whereas DAMGO elicits robust phosphorylation and internalization of MOR, morphine induces little MOR phosphorylation (Zhang et al, 1998; Koch et al, 2001). Upon overexpression of GRK2, morphine gains the capacity to induce MOR phosphorylation and internalization (Zhang et al, 1998).
Previously, we have reported that prolonged exposure to both DAMGO and morphine promotes desensitization of MOR signaling in transfected cells (Koch et al, 1998). Upon removal of the drug, DAMGO-desensitized receptors regained functional activity within minutes. In contrast, morphine-desensitized receptors failed to resensitize (Koch et al, 2001). Based on these results, we proposed that regulated endocytosis of MOR is required for its resensitization, and that the inability of morphine to cause substantial receptor endocytosis and recycling may facilitate the development of tolerance upon chronic drug exposure (Koch et al, 1998, 2001).
Recent evidence suggests that DAMGO-induced internalization of MOR is regulated by phosphorylation of several Ser/Thr residues including serine-363 (Ser363), Thr370 and Ser375 of the COOH-terminal tail (El Kouhen et al, 2001). Here, we show that exposure of MOR to morphine selectively induces the phosphorylation of Ser375 (corresponds to Ser377 of human MOR). In contrast to DAMGO, morphine-induced phosphorylation of Ser375 is sustained for prolonged periods and, hence, promotes persistent desensitization of MOR.
Results
First, we assessed whole-cell receptor phosphorylation in MOR-expressing HEK 293 cells in response to both DAMGO and morphine. DAMGO induced a rapid and robust phosphorylation of MOR (∼4-fold over basal). In contrast, the extent of morphine-induced phosphorylation of MOR reached only 32±6% (n=3) of DAMGO-induced phosphorylation (Figure 1). Mutation of Ser375 to Ala (S375A) reduced DAMGO-induced phosphorylation to 43±4% (n=3) of wild-type MOR and completely diminished morphine-induced phosphorylation (6±4%, n=3; Figure 1). These results indicate that Ser375 is a primary phosphoacceptor site for the DAMGO-activated MOR. However, the residual DAMGO-induced phosphorylation of the S375A mutant suggests that the DAMGO-activated MOR acquires a conformation, which also allows phosphorylation of several other Ser/Thr residues in addition to Ser375. In contrast, the morphine-activated MOR undergoes selective phosphorylation of Ser375.
Figure 1.
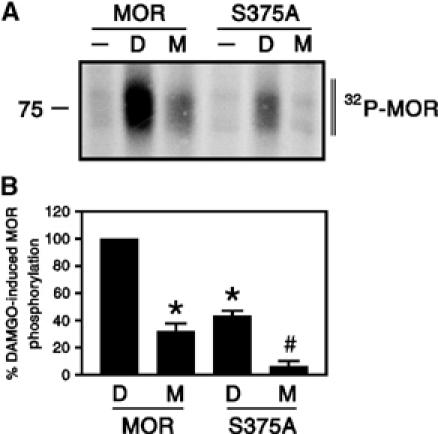
Agonist-selective phosphorylation of MOR and its S375A mutant. HEK 293 cells expressing HA-tagged MOR or HA-tagged S375A receptors were exposed to 10 μM DAMGO or 10 μM morphine for 30 min, and whole-cell receptor phosphorylation was determined as described under ‘Materials and methods'. (A) Representative autoradiographs from one of three independent experiments are shown. (B) Mean±s.e. of three independent experiments quantified by phosphorimager analysis. Data were normalized to DAMGO-induced phosphorylation of MOR. Results were analyzed by two-tailed Student's paired t-test (*P<0.05 versus DAMGO-induced phosphorylation of MOR; #P<0.05 versus morphine-induced phosphorylation of MOR). Note, (I) morphine-induced phosphorylation of MOR was significantly less than DAMGO-induced phosphorylation, (II) mutation of Ser375 to Ala (S375A) strongly reduced DAMGO-induced phosphorylation and completely diminished morphine-induced phosphorylation. The positions of molecular mass markers are indicated on the left (in kDa).
We next examined agonist-dependent Ser375 phosphorylation using an antibody that selectively recognizes the Ser375-phosphorylated form of MOR (see Supplementary data Figure 1). We found that Ser375 phosphorylation was at or near the detection limit in the basal state (Figure 2A). Exposure of MOR-expressing HEK 293 cells to 10 μM DAMGO for 30 min induced a robust phosphorylation of Ser375. Exposure to 10 μM morphine for 30 min also induced a clearly detectable Ser375 phosphorylation, however, to a much lesser extent (35±8%, n=6) than that observed with DAMGO (Figure 2A). Quantitative analysis of receptor internalization revealed that DAMGO-activated MOR receptors were efficiently removed from the cell surface, whereas morphine failed to cause substantial receptor endocytosis (Figure 2B). Overexpression of GRK2 strongly enhanced the level of morphine-induced Ser375 phosphorylation and internalization of MOR (Figure 2A and B, see Supplementary data Figure 2). Mutation of Ser375 to Ala (S375A) completely diminished detectable phospho-Ser375 immunoreactivity and strongly reduced agonist-driven receptor endocytosis (Figures 2A, B and 3). Confocal microscopy revealed that Ser375-phosphorylated MOR receptors were detectable at the plasma membrane within 2 min of DAMGO treatment (see Supplementary data Figure 3). In cells treated for 30 min with DAMGO, the majority of Ser375-phosphorylated MOR receptors were confined to clusters of intracellular vesicles presumably representing the perinuclear recycling compartment (Figure 3, see Supplementary data Figure 3). In contrast, in cells treated for 30 min with morphine, the majority of Ser375-phosphorylated MOR receptors were seen as punctate staining at the plasma membrane (Figure 3). These results provide direct evidence for agonist-induced phosphorylation of Ser375 within the carboxyl-terminal portion of the receptor, and suggest a direct relationship between Ser375 phosphorylation and internalization of MOR. Morphine appears to restrain the receptor in a conformation that is a poor substrate for GRK2-mediated phosphorylation of Ser375.
Figure 2.
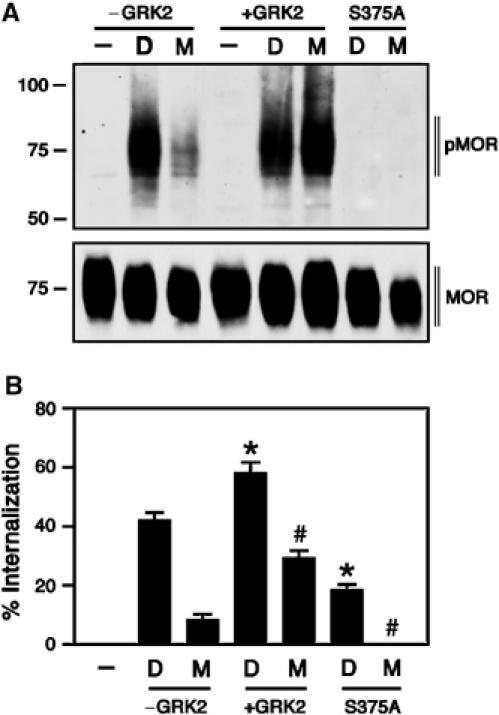
Relationship between Ser375 phosphorylation and internalization of MOR. (A) HEK 293 cells expressing the wild-type MOR or the S375A mutant were transiently transfected with GRK2 (+GRK2) or empty vector (−GRK2). After 2 days, cells were either not treated (−) or treated with 10 μM DAMGO (D) or 10 μM morphine (M) for 30 min, lysed and immunoblotted with an antibody specific for the Ser375-phosphorylated MOR (pMOR, upper panel). Expression of HA-tagged MOR and HA-tagged S375A receptors was confirmed by immunoblotting with an anti-HA antibody (MOR, lower panel). Note, (I) Ser375 phosphorylation was not detectable in untreated cells, (II) the morphine-induced Ser375 phosphorylation was ∼35% of DAMGO-induced phosphorylation, (III) overexpression of GRK2 strongly enhanced the morphine-induced Ser375 phosphorylation and (IV) phospho-Ser375 immunoreactivity was not detectable in cells expressing the S375A mutant. The positions of molecular mass markers are indicated on the left (in kDa). Three additional experiments gave similar results. (B) HEK 293 cells expressing the wild-type MOR or the S375A mutant were transiently transfected with GRK2 (+GRK2) or empty vector (−GRK2). After 2 days, cells were either not treated (−) or treated with 10 μM DAMGO (D) or 10 μM morphine (M) for 30 min. Cell surface receptors were labeled with rabbit anti-HA antibodies, followed by a peroxidase-conjugated secondary antibody. Receptor sequestration, quantified as the percent loss of cell-surface receptors in agonist-treated cells, was measured by ELISA. Data are presented as mean±s.e. of five independent experiments performed in quadruplicate. The results were analyzed by two-tailed Student's paired t-test (*P<0.05 versus DAMGO (−GRK2); #P<0.05 versus morphine (−GRK2)). Note, (I) in contrast to DAMGO, morphine failed to cause substantial receptor endocytosis, (II) overexpression of GRK2 strongly enhanced the level of morphine-induced internalization and (III) mutation of Ser375 to Ala (S375A) strongly reduced agonist-driven MOR endocytosis.
Figure 3.
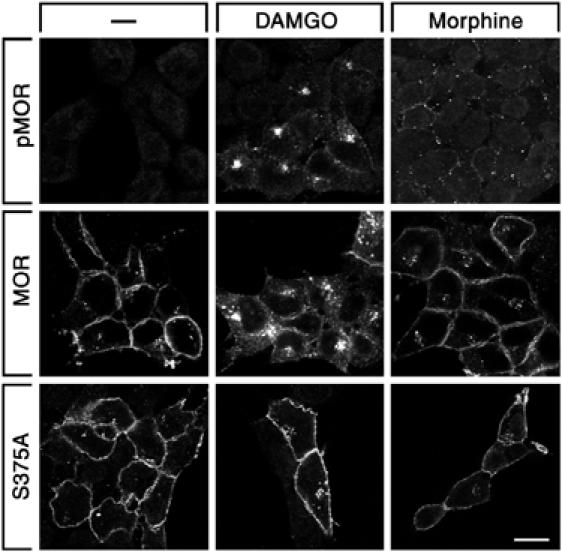
Agonist-selective internalization of Ser375-phosphorylated MOR receptors. HEK 293 cells expressing MOR or S375A were either not exposed (−) or exposed to 10 μM DAMGO or 10 μM morphine for 30 min, immunofluorescently labeled with anti-phospho-Ser375 (pMOR, upper panel) or anti-HA (MOR, middle and lower panels) antibodies and examined under a confocal microscope. Note, (I) in DAMGO-treated cells, the majority of Ser375-phosphorylated MOR receptors were confined to perinuclear clusters of vesicles, whereas, in morphine-treated cells, the majority of Ser375-phosphorylated MOR receptors was seen as punctate staining at the plasma membrane, (II) mutation of Ser375 to Ala (S375A) strongly reduced agonist-driven MOR endocytosis. Representative images from one of four independent experiments performed in duplicate are shown. Scale bar, 20 μm.
We then examined the dose–response relationship of various opioids to induce Ser375 phosphorylation and internalization of MOR (see Supplementary data Figure 4). The full agonists DAMGO, sufentanil and etorphine were potent inducers of both Ser375 phosphorylation and internalization (Figure 4A). Although morphine was more effective than the partial agonist buprenorphine, it induced Ser375 phosphorylation and internalization to a much lesser extent than full agonists (Figure 4A). In the presence of DAMGO or sufentanil, morphine behaved like the partial agonist buprenorphine and potently inhibited both Ser375 phosphorylation and internalization of MOR (Figure 4B). We also examined Ser375 phosphorylation and internalization after coapplication of morphine with low concentrations of DAMGO. However, none of the combinations of both drugs was able to increase the levels of Ser375 phosphorylation and internalization above those observed with morphine alone (see Supplementary data Figure 5). These results indicate that morphine exhibits clear partial agonistic properties.
Figure 4.
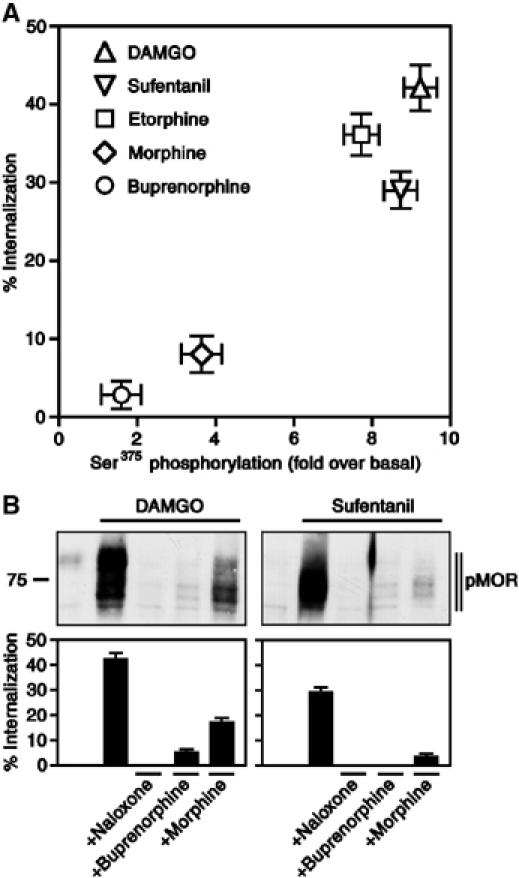
Partial agonistic properties of morphine. (A) HEK 293 cells expressing MOR were treated with saturating concentrations of DAMGO (10 μM), sufentanil (0.1 μM), etorphine (0.1 μM), morphine (10 μM) and buprenorphine (1 μM) for 30 min. For quantitative determination of receptor phosphorylation, cells were lysed and immunoblotted with an antibody specific for the Ser375-phosphorylated MOR. The levels of Ser375-phosphorylated MOR were quantified by densitometric analysis. Data were normalized to total MOR and expressed as the fold Ser375 phosphorylation over basal in untreated cells. Values represent means±s.e. of three to five independent experiments. For quantitative determination of receptor endocytosis, cell surface receptors were labeled with anti-HA antibodies, followed by a peroxidase-conjugated secondary antibody. Receptor sequestration, quantified as the percent loss of cell-surface receptors in agonist-treated cells, was measured by ELISA. Data are presented as mean±s.e. of three to five independent experiments performed in quadruplicate. Data for Ser375 phosphorylation and internalization were analyzed for correlation by the two-tailed Pearson test (r2=0.9057; P<0.05). Note, morphine was much less potent than the full agonists DAMGO, sufentanil and etorphine, but more potent than the partial agonist buprenorphine. (B) HEK 293 cells expressing MOR were treated with 10 μM DAMGO (left panel) or 0.1 μM sufentanil (right panel) in the presence or absence of 1 μM naloxone, 1 μM buprenorphine or 10 μM morphine for 30 min. For determination of receptor phosphorylation, cells were lysed and immunoblotted with an antibody specific for the Ser375-phosphorylated MOR (pMOR). The position of molecular mass marker is indicated on the left (in kDa). Three additional experiments gave similar results. For determination of receptor endocytosis, cell surface receptors were labeled with anti-HA antibodies followed by a peroxidase-conjugated secondary antibody. Receptor sequestration, quantified as the percent loss of cell-surface receptors in agonist-treated cells, was measured by ELISA. Data are presented as mean±s.e. of three independent experiments performed in quadruplicate. Note, morphine behaved like the partial agonist buprenorphine and potently inhibited both Ser375 phosphorylation and internalization of DAMGO- and sufentanil-activated MOR.
Recent studies suggest that, when compared with DAMGO, the relative efficacy of morphine to promote rapid desensitization and internalization of MOR is much less than its relative efficacy to activate G proteins (Yu et al, 1997; Alvarez et al, 2002; Borgland et al, 2003). We then examined the time course of Ser375 phosphorylation after exposure of MOR-expressing cells to either DAMGO or morphine. DAMGO stimulated a robust phosphorylation of Ser375 within 2 min, which remained at high levels throughout the 30-min treatment period (Figure 5, left panel). In contrast, morphine-induced Ser375 phosphorylation was first detectable after 5 min and increased steadily throughout the 30-min treatment period (Figure 5, left panel). Thus, the delayed onset of morphine-induced Ser375 phosphorylation may provide a potential explanation for its relatively poor efficacy to induce rapid desensitization of MOR signaling.
Figure 5.
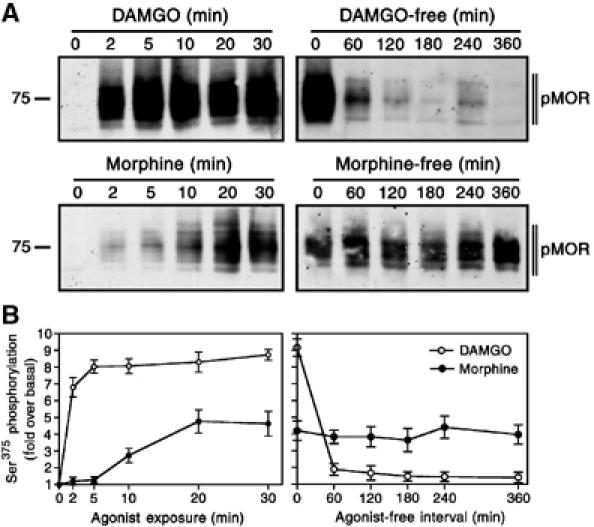
Time course of agonist-induced Ser375 phosphorylation and dephosphorylation. Left panel, HEK 293 cells expressing MOR were treated with 10 μM DAMGO or 10 μM morphine for either 0, 2, 5, 10, 20 or 30 min. Right panel, HEK 293 cells expressing MOR were treated with 10 μM DAMGO or 10 μM morphine for 30 min. Cells were extensively washed, incubated in the absence of agonist for either 0, 60, 120, 180, 240 or 360 min, lysed and immunoblotted with an antibody specific for the Ser375-phosphorylated MOR (pMOR). (A) Representative immunoblots from one of three independent experiments are shown. (B) Mean±s.e. of three independent experiments quantified by densitometric analysis. Data were expressed as the fold Ser375 phosphorylation over basal in untreated cells. The position of molecular mass markers is indicated on the left (in kDa).
The functional recovery of desensitized MORs involves dephosphorylation and recycling of internalized receptors to the plasma membrane (Koch et al, 1998). Upon removal of the drug, the majority of DAMGO-desensitized receptors was dephosphorylated within 60 min (Figure 5, right panel). Such a rapid dephosphorylation was also observed in sufentanil-treated cells (not shown). In contrast, morphine-desensitized receptors remained in a Ser375-phosphorylated state for at least 360 min in the absence of agonist (Figure 5, right panel). These results indicate that morphine-desensitized MOR receptors resided at the plasma membrane for prolonged periods and were not able to enter the recycling pathway.
We next examined the role of Ser375 phosphorylation in agonist-induced desensitization of MOR signaling to both adenylate cyclase and extracellular signal-regulated kinases 1 and 2 (ERK1/2). Prolonged exposure of cells expressing either MOR or its S375A mutant to DAMGO induced a time-dependent desensitization of opioid-induced adenylate cyclase inhibition and ERK1/2 activation (Figure 6A, C and E). The desensitization of mitogenic signaling was homologous because the lysophosphatidic acid (LPA) receptor, which is endogenously expressed in these cells, was still able to activate ERK1/2 after 240 min DAMGO exposure (Figure 6C and E). Prolonged exposure of MOR-expressing cells to morphine also induced a clearly detectable time-dependent desensitization of opioid-induced adenylate cyclase inhibition and ERK1/2 activation (Figure 6B, D and F). In contrast, the S375A mutant did not show any detectable morphine-induced desensitization of adenylate cyclase inhibition or ERK1/2 signaling during the 240-min treatment period (Figure 6B, D and F). These findings indicate that Ser375 phosphorylation is both sufficient and required for morphine-induced MOR desensitization. In addition, upon removal of the drug, DAMGO-desensitized receptors regained their ability to inhibit adenylate cyclase within 60 min (Figure 7). In contrast, morphine-desensitized receptors failed to resensitize during this time period, indicating that morphine promotes a persistent desensitization of MOR signaling (Figure 7).
Figure 6.
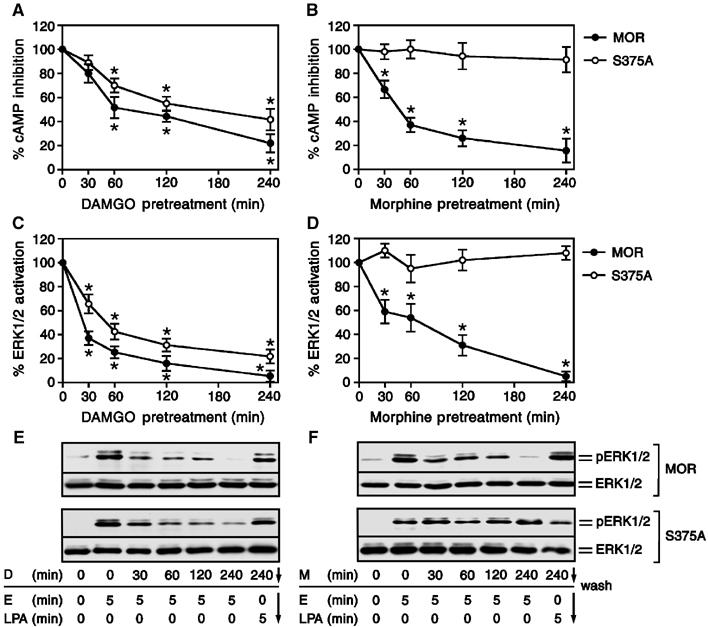
Role of Ser375 phosphorylation in morphine-induced desensitization of MOR. (A, B) HEK 293 cells expressing the wild-type MOR or the S375A mutant were treated with 10 μM DAMGO or 10 μM morphine for 0, 30, 60, 120 and 240 min. After washing, the cells were treated with either 25 μM forskolin or 25 μM forskolin plus 0.1 μM etonitazene, and cAMP levels were determined as described under ‘Materials and methods'. The maximum etonitazene-induced inhibition of forskolin-induced cAMP accumulation (for MOR-expressing cells 87±6%; for S375A-expressing cells 75±8%) without agonist preincubation has been defined as 100%. The values represent means±s.e. from three separate measurements performed in duplicate. * indicates a significant difference (P<0.05) between cells preincubated with DAMGO or morphine and cells that had not been preincubated (two-tailed Student's paired t-test). (C–F) HEK 293 cells expressing the wild-type MOR or the S375A mutant were treated with 10 μM DAMGO (D) or 10 μM morphine (M) for 0, 30, 60, 120 and 240 min. Cells were extensively washed and then exposed to 0.1 μM etonitazene (E) or 1 μM LPA for 5 min. Cells were lysed, equal amounts of protein were resolved by SDS–PAGE and levels of total ERK1/2 and phosphorylated ERK1/2 were determined by immunoblotting. (C, D) Results were quantified by densitometric analysis and normalized to total ERK1/2. For MOR and S375A, the maximum etorphine-stimulated ERK1/2 activation in cells that had not been preincubated was defined as 100%. Values represent means±s.e. of three independent experiments performed in duplicate. * indicates a significant difference (P<0.05) between cells preincubated with DAMGO or morphine and cells that had not been preincubated (two-tailed Student's paired t-test). (E, F) representative immunoblots for the wild type MOR (upper panel) and the S375A (lower panel) mutant. The positions of phospho-ERK1/2 (pERK1/2) and total ERK1/2 (ERK1/2) are indicated on the right. Note, exchange of Ser375 to Ala completely diminished morphine-induced desensitization of opioid-induced MOR signaling.
Figure 7.
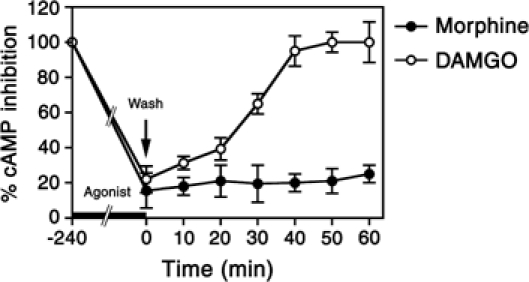
Differential resensitization of DAMGO- and morphine-desensitized MOR receptors. MOR-expressing HEK 293 cells were treated with 10 μM DAMGO or 10 μM morphine for 240 min. Cells were extensively washed and incubated in the absence of agonist for either 0, 10, 20, 30, 40, 50 or 60 min. Cells were then treated with either 25 μM forskolin or 25 μM forskolin plus 0.1 μM etonitazene, and cAMP levels were determined as described under ‘Materials and methods'. The maximum etonitazene-induced inhibition of forskolin-induced cAMP accumulation without agonist preincubation has been defined as 100%. The values represent means±s.e. from three separate measurements performed in duplicate.
To ensure that Ser375 phosphorylation of MOR was not an artifact of the HEK 293 cell model, we examined whether this regulation also occurred in cultured neurons. Cortical neuron cultures were prepared from embryonic rats, transfected with MOR and allowed to mature for 1 week. Similar to that observed in HEK 293 cells, Ser375-phosphorylated MOR receptors were detectable at the plasma membrane within 2 min of DAMGO treatment (Figure 8). In neurons treated for 30 min with DAMGO, nearly all detectable Ser375-phosphorylated MOR receptors were confined to intracellular vesicles (Figure 8). In contrast, in neurons treated for 30 min with morphine, the majority of Ser375-phosphorylated MOR receptors were seen as punctate staining at the plasma membrane (Figure 8). These results strongly indicate that agonist-dependent Ser375 phosphorylation also occurs in intact cortical neurons, and that the trafficking of Ser375-phosphorylated MOR receptors is also regulated in an agonist-selective manner in these neurons.
Figure 8.
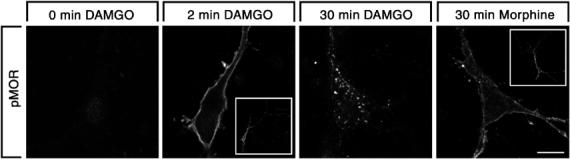
Agonist-induced Ser375 phosphorylation of MOR in cultured neurons. Cortical neuron cultures were prepared from embryonic rats at embryonic day 18, transfected with MOR and allowed to mature for 1 week. Neurons were treated with 10 μM DAMGO or 10 μM morphine for 0, 2 or 30 min. Cells were then immunofluorescently labeled with anti-phospho-Ser375 antibodies and examined under a confocal microscope. Note, (I) in DAMGO-treated cells, the majority of Ser375-phosphorylated MOR receptors were confined to perinuclear clusters of vesicles after 30 min, whereas, in morphine-treated cells, the majority of Ser375-phosphorylated MOR receptors resided at the plasma membrane. Representative images from one of two independent experiments performed in duplicate are shown. Scale bar, 20 μm.
Discussion
At present, divergent views exist concerning the extent to which either opioid receptor desensitization or adenylate cyclase superactivation contribute to the development of tolerance to morphine. The receptor activity versus endocytosis (RAVE) model proposes that morphine may induce adenylate cyclase superactivation to a greater extent than other opioids, which in turn exacerbates the development of tolerance (Whistler et al, 1999; Finn and Whistler, 2001). In contrast, studies using mice lacking β-arrestin-2 show that opioid receptor desensitization directly contributes to tolerance and that tolerance and adenylyl cyclase superactivation are two dissociable phenomena (Bohn et al, 1999, 2000). Here, we propose that morphine is unique in that it promotes terminal opioid receptor desensitization by inducing a sustained phosphorylation of Ser375. Morphine-desensitized receptors remain at the plasma membrane in a Ser375-phosphorylated state for prolonged periods and are not able to enter the recycling pathway. This hypothesis is compatible with several hallmarks of opioid tolerance. First, morphine fails to promote significant downregulation of MOR even under conditions that induce profound cellular tolerance (Lenoir et al, 1984; Simantov et al, 1984). Second, when administered chronically at equieffective analgesic doses, etorphine and methadone, which are potent inducers of MOR internalization and recycling, produce less tolerance than morphine (Rezvani et al, 1983; Duttaroy and Yoburn, 1995; Mercadante et al, 1998). Third, upon withdrawal from morphine, reversal of the tolerant state requires days to weeks (Grecksch et al, 1974). Such a considerably long period of time would also be expected for new receptor synthesis to occur.
We show that morphine induces Ser375 phosphorylation in cultured cortical neurons, and that Ser375-phosphorylated MOR receptors remain at the plasma membrane in these neurons. Previous studies have reported that morphine does not produce detectable internalization of MOR in neural tissues except for dendrites of nucleus accumbens neurons (Haberstock-Debic et al, 2003). We also show that overexpression of GRK2 converts morphine into a potent inducer of Ser375 phosphorylation and internalization, suggesting that the cellular complement of GRK2 in many central neurons is not sufficient to drive morphine-induced internalization of MOR.
It has recently been reported that subanalgesic doses of DAMGO can prevent morphine tolerance by facilitating the internalization of morphine-activated MOR receptor complexes (He et al, 2002). Although this is an intriguing finding, we and others (Bailey et al, 2003) failed to provide evidence for synergistic effects of DAMGO and morphine. In contrast, we defined morphine as partial agonist by its ability to inhibit DAMGO- and sufentanil-induced phosphorylation and internalization of MOR. Thus, the mechanistic basis by which DAMGO and morphine may interact to prevent the development of morphine tolerance remains unknown.
In conclusion, we provide evidence for the partial agonistic properties of morphine. As a partial agonist, morphine activates the receptor and restrains it in a conformation that is a poor substrate for GRK2-mediated phosphorylation. Morphine induces selectively the phosphorylation of carboxy-terminal Ser375, which is sufficient to drive receptor desensitization but not sufficient to drive receptor endocytosis. Hence, morphine-desensitized receptors remain at the plasma membrane in a Ser375-phosphorylated state for prolonged periods and are not able to enter the recycling pathway. We propose that sustained Ser375 phosphorylation induces persistent desensitization of MOR, which in turn facilitates the development of cellular tolerance.
Materials and methods
Cell lines
HEK 293 cells stably expressing the HA-tagged MOR (Bmax 778±6 fmol/mg of membrane protein; KD 1.55±0.3 nM) or the HA-tagged S375A mutant (Bmax 624±18 fmol/mg of membrane protein; KD 1.32±0.22 nM) were generated and characterized using radioligand binding assays, Western blot analysis and immunocytochemistry as described elsewhere (Koch et al, 2001; Pfeiffer et al, 2002). Transient transfections were carried out using Lipofectamine 2000 (Invitrogen, Karlsruhe, Germany).
Whole-cell phosphorylation assays
Phosphorylation studies were carried out as described (Koch et al, 2001; Pfeiffer et al, 2002). After agonist incubation, [32P]orthophosphate-labeled cells were scraped into radioimmune precipitation assay buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 10 mM NaF, 10 mM disodium pyrophosphate, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 0.2 mM phenylmethylsulfonylfluoride, 10 μg/ml leupeptin, 1 μg/ml pepstatin A, 1 μg/ml aprotinin and 10 μg/ml bacitracin) and solubilized. Immunoprecipitations were carried out using 50 μl of HA affinity beads (Covance, Berkeley, CA). Immunocomplexes were eluted from the beads using SDS-sample buffer for 20 min at 56°C. Samples were size-separated by 8% SDS gels followed by autoradiography. The extent of receptor phosphorylation was quantitated using a Fuji PhosphorImaging system and BAS 1000 software. Loading of equal amounts of receptor proteins in each lane was confirmed by Western blot analysis.
Drug treatment
In dose–response studies, cells were treated with 1, 10 or 100 μM DAMGO; 1, 10 or 100 μM morphine; 0.01, 0.1 or 1 μM sufentanil; 0.01, 0.1 or 1 μM etorphine and 0.1, 1 or 10 μM buprenorphine for 30 min. Based on their reported relative binding affinities (Lee et al, 1999), the various compounds are expected to reach similar saturating concentrations under these conditions. In time-course studies, cells were exposed to either 10 μM DAMGO, 10 μM morphine or 0.1 μM sufentanil for 0, 2, 5, 10, 20 or 30 min, washed three times with PBS and incubated in culture medium without agonist for 0, 60, 120, 180, 240 or 360 min.
Western blotting
Cells were lysed in radioimmune precipitation assay buffer, and glycosylated proteins were partially enriched using wheat-germ-lectin agarose beads (Amersham, Braunschweig, Germany) as described (Koch et al, 2001). Proteins were eluted from the beads using SDS-sample buffer for 20 min at 60°C, and then resolved on 8% SDS–polyacrylamide gels. After electroblotting, the membranes were incubated with anti-phospho-Ser375 antibody (1:1000; Cell Signaling Technology, Beverly, MA) or rabbit anti-HA antibody (0.5 μg/ml; Gramsch Laboratories, Schwabhausen, Germany), followed by detection using an enhanced chemiluminescence detection system (Amersham).
Immunocytochemistry
Cells were grown onto poly-L-lysine-coated coverslips overnight. After agonist exposure, cells were fixed, permeabilized and incubated with anti-phospho-Ser375 antibody (1:200; Cell Signaling Technology) or rat anti-HA antibody (1:1000; Roche, Mannheim, Germany) for 16 h at room temperature, followed by detection using a cyanine 3.18-conjugated secondary antibody (1:200; Jackson ImmunoResearch, West Grove, PA). Cells were then dehydrated, permanently mounted and examined using a Leica TCS-NT confocal microscope.
Internalization assays
Cells were seeded onto 24-well plates. On the next day, cells were preincubated with 1 μg anti-HA antibody for 2 h in OPTIMEM 1 (Invitrogen) at 4°C. Cells were then exposed to agonist, fixed and developed with peroxidase-conjugated secondary antibody as described (Pfeiffer et al, 2002).
cAMP assays
Cells were seeded onto 12-well dishes. On the next day, cells were treated with 10 μM DAMGO or 10 μM morphine for 0, 30, 60, 120 or 240 min, washed three times in PBS and then incubated in medium containing 25 μM forskolin or 25 μM forskolin plus 0.1 μM etonitazene for 15 min at 37°C. Incubation was terminated by removal of the culture medium and subsequent addition of 1 ml of ice-cold HCl/ethanol (1 volume of 1 N HCl with 100 volumes of ethanol). After centrifugation, the supernatant was evaporated, the residue was dissolved in TE buffer (50 mM Tris–EDTA, pH 7.5) and the cAMP content was determined using a commercially available radioimmunoassay kit (Amersham).
ERK assays
Cells were seeded onto 24-well dishes, grown in DMEM medium containing 0.5% FCS overnight. Cells were then treated with 10 μM DAMGO or 10 μM morphine for 0, 30, 60, 120 or 240 min, washed three times in PBS and then exposed to 0.1 μM etonitazene or 10 μM LPA for 5 min at 37°C. Incubation was terminated by removal of the culture medium, and subsequent addition of 150 μl boiling SDS-sample buffer. The samples were assayed by Western blotting as described previously (Pfeiffer et al, 2002).
Neuronal cultures
Neurons were prepared from cerebral cortices of embryonic Sprague–Dawley rats at embryonic day 18 as described (Voigt et al, 2001). Cells were transfected with MOR using a Nucleofector (Amaxa, Köln, Germany), and then cultivated on poly-D-lysine-coated coverslips in glial-conditioned medium. After 1 week, neurons were treated with 10 μM DAMGO or 10 μM morphine for 0, 2 or 30 min. Cells were were fixed, permeabilized and incubated with anti-phospho-Ser375 antibody (1:200; Cell Signaling Technology) for 16 h at room temperature, followed by detection using a cyanine 3.18-conjugated secondary antibody (1:200; Jackson ImmunoResearch). Cells were then dehydrated, permanently mounted and examined using a Leica TCS-NT confocal microscope.
Data analysis
NIH Image 1.62 software was used to densitize and quantify phospho-Ser375 and phospho-ERK1/2 levels. Statistical analysis was carried out with the Student's t-test and the Pearson test using GraphPad Prism 3.0 software. P-values <0.05 were considered as statistically significant.
Supplementary Material
Supplementary Information
Acknowledgments
We thank PY Law for plasmid encoding the S375A mutant and MG Caron for plasmid encoding GRK2. This work was supported by Deutsche Forschungsgemeinschaft Grants SCHU 924/4-4 (to SS) and SFB426 TPA2 (to VH).
References
- Alvarez VA, Arttamangkul S, Dang V, Salem A, Whistler JL, Von Zastrow M, Grandy DK, Williams JT (2002) mu-Opioid receptors: ligand-dependent activation of potassium conductance, desensitization, and internalization. J Neurosci 22: 5769–5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Couch D, Johnson E, Griffiths K, Kelly E, Henderson G (2003) Mu-opioid receptor desensitization in mature rat neurons: lack of interaction between DAMGO and morphine. J Neurosci 23: 10515–10520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG (2000) Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 408: 720–723 [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT (1999) Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286: 2495–2498 [DOI] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ (2003) Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem 278: 18776–18784 [DOI] [PubMed] [Google Scholar]
- Duttaroy A, Yoburn BC (1995) The effect of intrinsic efficacy on opioid tolerance. Anesthesiology 82: 1226–1236 [DOI] [PubMed] [Google Scholar]
- El Kouhen R, Burd AL, Erickson-Herbrandson LJ, Chang CY, Law PY, Loh HH (2001) Phosphorylation of Ser363, Thr370, and Ser375 residues within the carboxyl tail differentially regulates mu-opioid receptor internalization. J Biol Chem 276: 12774–12780 [DOI] [PubMed] [Google Scholar]
- Finn AK, Whistler JL (2001) Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron 32: 829–839 [DOI] [PubMed] [Google Scholar]
- Grecksch G, Ott T, Matthies H (1974) Effects of orotic acid on the development of morphine tolerance. Psychopharmacologia 36: 337–346 [DOI] [PubMed] [Google Scholar]
- Haberstock-Debic H, Wein M, Barrot M, Colago EE, Rahman Z, Neve RL, Pickel VM, Nestler EJ, von Zastrow M, Svingos AL (2003) Morphine acutely regulates opioid receptor trafficking selectively in dendrites of nucleus accumbens neurons. J Neurosci 23: 4324–4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Fong J, von Zastrow M, Whistler JL (2002) Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell 108: 271–282 [DOI] [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M (1996) Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem 271: 19021–19024 [DOI] [PubMed] [Google Scholar]
- Koch T, Schulz S, Pfeiffer M, Klutzny M, Schroder H, Kahl E, Hollt V (2001) C-terminal splice variants of the mouse mu-opioid receptor differ in morphine-induced internalization and receptor resensitization. J Biol Chem 276: 31408–31414 [DOI] [PubMed] [Google Scholar]
- Koch T, Schulz S, Schroder H, Wolf R, Raulf E, Hollt V (1998) Carboxyl-terminal splicing of the rat mu opioid receptor modulates agonist-mediated internalization and receptor resensitization. J Biol Chem 273: 13652–13657 [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE (1998) Neuroscience of addiction. Neuron 21: 467–476 [DOI] [PubMed] [Google Scholar]
- Lee KO, Akil H, Woods JH, Traynor JR (1999) Differential binding properties of oripavines at cloned mu- and delta-opioid receptors. Eur J Pharmacol 378: 323–330 [DOI] [PubMed] [Google Scholar]
- Lenoir D, Barg J, Simantov R (1984) Characterization and down-regulation of opiate receptors in aggregating fetal rat brain cells. Brain Res 304: 285–290 [DOI] [PubMed] [Google Scholar]
- Mercadante S, Casuccio A, Agnello A, Serretta R, Calderone L, Barresi L (1998) Morphine versus methadone in the pain treatment of advanced-cancer patients followed up at home. J Clin Oncol 16: 3656–3661 [DOI] [PubMed] [Google Scholar]
- Nestler EJ (1996) Under siege: the brain on opiates. Neuron 16: 897–900 [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK (1997) Molecular and cellular basis of addiction. Science 278: 58–63 [DOI] [PubMed] [Google Scholar]
- Pfeiffer M, Koch T, Schroder H, Laugsch M, Hollt V, Schulz S (2002) Heterodimerization of somatostatin and opioid receptors cross-modulates phosphorylation, internalization, and desensitization. J Biol Chem 277: 19762–19772 [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ (1998) G protein-coupled receptor kinases. Annu Rev Biochem 67: 653–692 [DOI] [PubMed] [Google Scholar]
- Rezvani A, Huidobro-Toro JP, Hu J, Way EL (1983) A rapid and simple method for the quantitative determination of tolerance development to opiates in the guinea-pig ileum in vitro. J Pharmacol Exp Ther 225: 251–255 [PubMed] [Google Scholar]
- Simantov R, Lotem J, Levy R (1984) Selectivity in the control of opiate receptor density in the animal and in cultured fetal brain cells. Neuropeptides 5: 197–200 [DOI] [PubMed] [Google Scholar]
- Voigt T, Opitz T, de Lima AD (2001) Synchronous oscillatory activity in immature cortical network is driven by GABAergic preplate neurons. J Neurosci 21: 8895–8905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M (1999) Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron 23: 737–746 [DOI] [PubMed] [Google Scholar]
- Yu Y, Zhang L, Yin X, Sun H, Uhl GR, Wang JB (1997) Mu opioid receptor phosphorylation, desensitization, and ligand efficacy. J Biol Chem 272: 28869–28874 [DOI] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG (1998) Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci USA 95: 7157–7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


