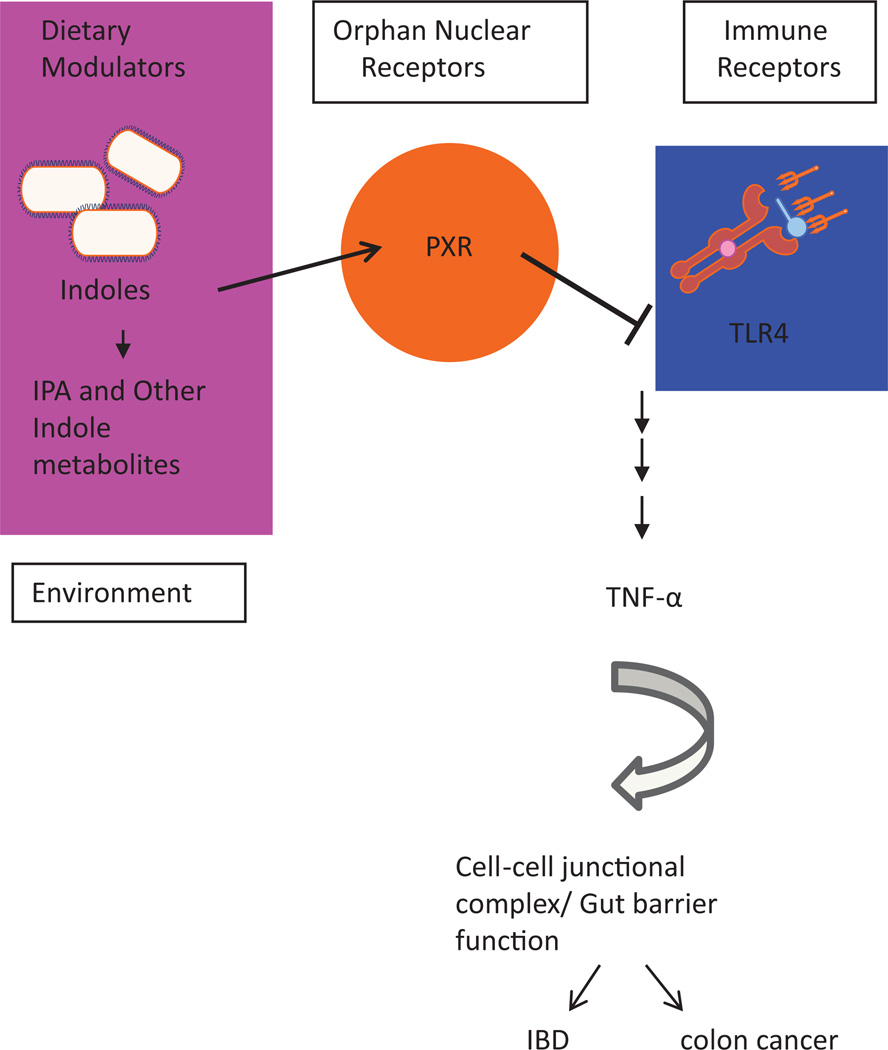
Figure 1.
We demonstrate that in the intestines, where PXR is expressed in intestinal epithelial cells in a crypt-villus gradient (apical IEC), in homeostasis, dietary tryptophan-derived bacterial metabolites (i.e. indole-3-propionic acid or IPA) tonically activate PXR and induce a down-regulation of TLR4, and its downstream signaling pathway. This results in modulating the abundance of TNF-α, which in turn modulates intestinal barrier function (i.e. permeability). In the context of preserved indole concentrations, loss of PXR (often observed in some Crohn’s ileitis and UC) will promote the inflammatory response. In corollary, with excess loss of dietary modulators (e.g., tryptophan/IPA), and/or specific indole metabolizing bacteria (e.g., antibiotics), there is increased permeability exacerbating underlying inflammatory pathology. In this model, restitution of signaling homeostasis, either by reconstituting intestinal loss of PXR or indole-metabolite producing bacteria and/or PXR activating bacterial metabolites (i.e. IPA via tryptophan catabolism), could result in abrogating pro-inflammatory signals and loss of barrier permeability in the context of intestinal inflammation.