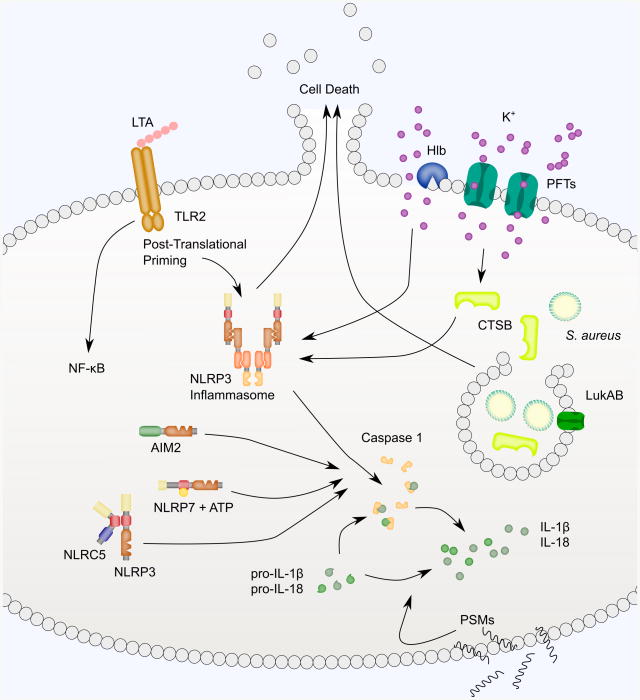
Fig. 2.
NLRP3, NLRC5, NLRP7, AIM2 and other inflammasome-like signaling is activated in response to S. aureus. Activation of inflammasome signaling typically proceeds in two steps. First, TLRs engage PAMPS to prime inflammasome signaling. S. aureus lipoteichoic acid (LTA), a major constituent of the cell wall of Gram-positive bacteria binds TLR2. TLR signaling activates NF-κB-mediated transcription of pro-IL-1β and also triggers post-translational modifications of the inflammasome signaling pathway. Activation of the NLRP3 inflammasome occurs through pore-mediated and phagocytosis-mediated processes. PFTs and Hlb damage the plasma membrane and cause potassium efflux necessary for inflammasome activation. PVL-induced NLRP3 inflammasome signaling requires CTSB activation. PVL, Hla and LukAB also promote Caspase 1-independent cell death. PFTs may also destabilize lysophagosomes during phagocytosis of S. aureus. In particular, LukAB has been shown to bind CD11b on the phagosomal membrane to promote S. aureus escape. This triggers NLRP3-dependent cytokine secretion and NLRP3-independent cell death. Lysophagosomal rupture is thought to lead to CTSB leakage into the cytoplasm, though this has not been shown directly with S. aureus. A variety of other inflammasomes have also been implicated in sensing S. aureus. NLRC5 binds to NLRP3 and enhances cytokine secretion. NLRP7 and its ATP-binding activity are required for Caspase 1 activation in response to S. aureus. AIM2 is activated in response to S. aureus central nervous system abscess formation, though it isn't clear if AIM2 is sensing S. aureus or a danger-associated molecular pattern resulting from S. aureus infection. PSMs also trigger secretion of IL-1β and IL-18, but through a Caspase 1-independent mechanism.