Abstract
Background
To address low HPV vaccination coverage, the American Academy of Family Physicians (AAFP) and the American Academy of Pediatrics (AAP) have launched national campaigns encouraging physicians to deliver strong HPV vaccine recommendations. We surveyed family physicians and pediatricians to examine the impact of these efforts on physicians’ recommendation practices.
Methods
A national sample of family physicians and pediatricians (n=776) completed our online survey in 2014. The survey assessed reach, content, and influence of AAFP and AAP communications about HPV vaccination. The survey also assessed quality of physicians’ communication practices for recommending HPV vaccination.
Results
Forty-seven percent of family physicians reported receiving information on HPV vaccination from AAFP, while 62% of pediatricians reported receiving information from AAP. Among physicians reached by AAFP or AAP, most reported receiving the message to give strong recommendations to adolescent boys (71%) and girls (78%). Although receiving information was not associated with HPV vaccine recommendation quality, receiving the message to give strong recommendations correlated with delivering higher-quality recommendations for boys (OR=4.19, 95% CI, 2.64–6.64) and girls (OR=3.15, 95% CI, 1.91–5.18). Over half of physicians reported improving their HPV vaccine communication after receiving information from AAFP (69%) or AAP (53%).
Conclusions
Our findings suggest that it is important for AAFP and AAP to communicate the need for strong HPV vaccine recommendations. Given that many physicians reported improving their recommendation practices, professional organizations stand to contribute to increasing HPV vaccination coverage, but they will likely need to increase the intensity of quality improvement efforts to do so.
Keywords: HPV vaccine, adolescent health, health communication, medical education, professional organizations, healthcare quality improvement
INTRODUCTION
About 79 million Americans currently are infected with human papillomavirus (HPV), and about 14 million are newly infected each year.1 To prevent HPV infection, the Advisory Committee on Immunization Practices (ACIP) has recommended routine administration of the 3-dose HPV vaccine series to 11- to 12-year-olds since 2006 for girls and 2011 for boys.2 Despite these recommendations, only 42% of girls and 28% of boys, ages 13–17, had completed the HPV vaccine series as of 2015.3 This low level of coverage is far below the Healthy People 2020 goal of 80% as well as what has been achieved in other countries, including the United Kingdom, Australia, Mexico, and Rwanda.4 The Centers for Disease Control and Prevention (CDC) estimates that our failure to meet the Healthy People 2020 goal for HPV vaccination in the U.S. will lead to over 50,000 future cervical cancer cases for today’s population of girls ages 12 and younger.5 For every additional year of low coverage, another 4,400 girls will develop cervical cancer over the course of their lifetimes.5 Given the substantial health benefits of HPV vaccination, increasing vaccine uptake among girls and boys is a national priority.
Barriers to the delivery of HPV vaccine are complex, and include parental hesitancy, lack of awareness that the vaccine should be given to boys, and challenges related to the completion of the 3-dose series.6 Nevertheless, successful delivery of HPV vaccine still depends to a large extent on healthcare providers’ recommendation practices.7 Most adolescents who receive a provider’s recommendation for HPV vaccination are subsequently vaccinated.8–10 However, parental reports suggests that only about half of age-eligible adolescents receive recommendations,8,9 and only about one third of physicians recommend HPV vaccination for most of their 11- to 12-year-old patients.11 Provider characteristics associated with recommending HPV vaccination include physician specialty and knowledge and attitudes about HPV vaccinations.11 Consistent with other cancer prevention services, providers typically report that they are motivated to follow HPV vaccination guidelines of professional organizations, such as the American Academy of Family Physicians (AAFP) and the American Academy of Pediatrics (AAP).12–14 These organizations play a key role in developing and disseminating HPV vaccination guidelines and in providing opportunities for continuing medical education and immunization quality improvement. For example, AAFP and AAP have distributed “dear colleague” letters to physicians that urge them to “give a strong recommendation for HPV vaccine to increase uptake”.15 Professional organizations appear central to immunization quality improvement efforts, yet little is known about how their communications influence physicians’ HPV vaccine recommendations.
To address this gap, we sought to assess the reach, content, and influence of professional organizations’ communications about HPV vaccination. We hypothesized that among physicians who reported being reached by professional organizations, they would indicate greater awareness of HPV vaccination practice guidelines and be more likely to deliver guideline-consistent recommendations. By exploring the relationship between professional organizations’ communication and physicians’ recommendation practices, we sought to understand mechanisms for disseminating information about HPV vaccine to providers and promoting uptake among adolescents.
METHODS
Participants and procedures
The Physician Communication about HPV Vaccination Study was an online survey of U.S. family physicians and pediatricians conducted from April to June 2014. As described previously, respondents were members of an existing national panel of physicians maintained by a survey research company.16,17 Constructed using American Medical Association lists, the panel included similar numbers of family physicians (51%) and pediatricians (49%) from all regions of the U.S. (22% Northeast; 23% Midwest; 37% South; 18% West).18 Eligible physicians for our survey provided preventive care to patients ages 11 and 12 because national guidelines specify this age range for routine HPV vaccination.
Of 2,368 physicians invited by email to participate, 1,022 (43%) responded by accessing the survey website, of which three-quarters (76%) were eligible and completed the survey.16 We were unable to collect data on ineligible respondents, though a third (33%) of physicians in the panel completed the survey.16 Participants provided informed consent and received up to $45 for completing the survey.16 The University of North Carolina Institutional Review Board approved all procedures for this study.
Measures
Our survey assessed whether physicians reported having received information about HPV vaccines from six different sources with one item: “From which of these organizations have you directly received letters, emails, or other information about HPV vaccine in the last year?” Sources were: “Drug companies”; “Insurance companies”; “American Academy of Family Physicians (AAFP)”; “American Academy of Pediatrics (AAP)”; “the Centers for Disease Control and Prevention”; and the “President’s Cancer Panel.” For physicians who indicated receiving information from AAFP or AAP, a follow-up item assessed the content of that communication in terms of 5 messages: “HPV vaccination rates are too low”; “It is important to identify parents’ concerns before recommending HPV vaccine”; “My recommendation should be based on adolescents’ risk of getting HPV”; and “I should give a strong recommendation for HPV vaccine to all 11- to 12-year old [males/females].” A second follow-up item assessed the influence of the communication with the following response options: “Made me discuss it more often”; “Made me recommend it more often”; “Made me recommend it more strongly”; or “Had no effect.” Finally, two items assessed physicians’ knowledge of their professional organization’s position of supporting routine HPV vaccination for 11- and 12-year-old males and females. Response options were: “No position”; “Physicians can give HPV vaccine at their discretion”; “Physicians should routinely give them HPV vaccine”; or “Not sure.” These two items specified AAFP for family physicians and AAP for pediatricians.
To explore physicians’ recommendation practices, we assessed quality of their HPV vaccine communication using a measure that we have previously reported.16 This measure consisted of five quality indicators: timeliness for males (routinely recommending HPV vaccine by ages 11–12 as per national guidelines); timeliness for females; strength of endorsement (saying HPV vaccine is highly important for this age group); consistency (avoiding risk-based approaches to recommending HPV vaccine); and urgency (recommending same-day vaccination). We calculated recommendation quality by awarding each quality indicator one point for a total score ranging from 0 to 5. For our analysis, we categorized physicians as using “high quality” recommendation practices if they received a score of 4 or higher or “low quality” recommendation practices if they received a score of 3 or lower.
We collected respondents’ demographic and professional characteristics including: medical specialty; sex; years in practice since residency; number of adolescent patients seen in a typical week; and percentage of vaccine doses delivered through the Vaccines for Children (VFC) program, which provides free vaccines to vulnerable populations.19 For clinical characteristics, we assessed clinic type (private practice versus other), total number of physicians in the clinic, and state in which the clinic was located. We categorized clinic locations by U.S. region using U.S. Census classifications.20 The full survey instrument is available online (www.unc.edu/~ntbrewer/hpv.htm).
Statistical analysis
We used bivariate logistic regression to assess correlates of knowing professional organizations’ positions for routine HPV vaccination of 11- to 12-year-old males and females. We then entered statistically significant correlates into multivariate models. To assess associations between organizational communication and physicians’ HPV vaccine recommendation quality, we used chi-squared analyses. Specifically, we measured associations between physicians’ HPV vaccine recommendation quality and the following: receiving information from [AAFP/AAP]; receiving the message to give strong HPV vaccine recommendations for [males/females]; and knowing professional organizations’ position on HPV vaccination for [males/females]. We used Stata Version 13.0 (College Station, TX) to conduct our analyses, which were two-tailed with a critical alpha of 0.05.
RESULTS
Sample Characteristics
Our sample (n=776) consisted of 366 family physicians (47%) and 410 pediatricians (53%), of whom about two-thirds (68%) were male (Table 1).18 Most were in private practice (85%), worked in clinics with two or more physicians (85%), and saw over 10 adolescent patients per week (83%). Physicians’ practices were located in the Northeast (24%), Midwest (21%), South (35%), and West (20%) of the U.S.
Table 1.
Sample characteristics and correlates of knowing professional organizations’ positions on HPV vaccination (n=776).
| Overall | Position for 11–12 year old boys | Position for 11–12 year old girls | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Knew Position | Bivariate | Knew Position | Bivariate | |||||||
|
| ||||||||||
| n | (%) | n | (%) | OR | (95% CI) | n | (%) | OR | (95% CI) | |
| Physician characteristics | ||||||||||
| Medical specialty | ||||||||||
| Pediatrics | 410 | (53) | 348 | (85) | 1 | 368 | (90) | 1 | ||
| Family practice | 366 | (47) | 205 | (56) | 0.23 | (0.16–0.32)** | 241 | (66) | 0.22 | (0.15–0.32)** |
| Sex | ||||||||||
| Male | 526 | (68) | 358 | (68) | 1 | 402 | (76) | 1 | ||
| Female | 250 | (32) | 195 | (78) | 1.66 | (1.17–2.36)** | 207 | (83) | 1.48 | (1.01–2.18)* |
| Years in practice | ||||||||||
| ≤19 | 352 | (45) | 255 | (72) | 1 | 280 | (80) | 1 | ||
| ≥20 | 424 | (55) | 298 | (70) | 0.90 | (0.66–1.23) | 329 | (78) | 0.89 | (0.63–1.26) |
| Adolescent patients per week | ||||||||||
| ≤9 | 129 | (17) | 76 | (59) | 1 | 88 | (68) | 1 | ||
| 10–24 | 351 | (45) | 237 | (68) | 1.45 | (0.96–2.20) | 272 | (77) | 1.60 | (1.03–2.51)* |
| ≥25 | 296 | (38) | 240 | (81) | 2.99 | (1.89–4.71)** | 249 | (84) | 2.47 | (1.52–4.01)** |
| Vaccine doses through VFC | ||||||||||
| ≤9% | 290 | (37) | 185 | (64) | 1 | 210 | (72) | 1 | ||
| 10%–49% | 274 | (35) | 219 | (80) | 2.26 | (1.54–3.31)** | 235 | (86) | 2.30 | (1.50–3.51)** |
| ≥50% | 152 | (20) | 116 | (76) | 1.83 | (1.17–2.85)** | 125 | (82) | 1.76 | (1.08–2.88)* |
| Not sure | 60 | (8) | 33 | (55) | 0.69 | (0.40–1.22) | 39 | (65) | 0.71 | (0.39–1.28) |
| Clinic characteristics | ||||||||||
| Type | ||||||||||
| Private practice | 660 | (85) | 473 | (72) | 1 | 522 | (79) | 1 | ||
| Other | 116 | (15) | 80 | (69) | 0.88 | (0.57–1.35) | 87 | (75) | 0.79 | (0.50–1.26) |
| Total physicians | ||||||||||
| 1/solo | 115 | (15) | 70 | (61) | 1 | 82 | (71) | 1 | ||
| 1–4 | 283 | (36) | 195 | (69) | 1.42 | (0.91–2.24) | 218 | (77) | 1.35 | (0.83–2.20) |
| 5–9 | 217 | (28) | 166 | (77) | 2.09 | (1.28–3.41)** | 181 | (83) | 2.02 | (1.18–3.47)* |
| ≥10 | 161 | (21) | 122 | (76) | 2.01 | (1.20–3.38)** | 128 | (80) | 1.56 | (0.89–2.72) |
| Region | ||||||||||
| Northeast | 184 | (24) | 133 | (72) | 1 | 148 | (80) | 1 | ||
| Midwest | 165 | (21) | 112 | (68) | 0.81 | (0.51–1.28) | 123 | (75) | 0.71 | (0.43–1.18) |
| South | 275 | (35) | 201 | (73) | 1.04 | (0.69–1.58) | 222 | (81) | 1.02 | (0.64–1.63) |
| West | 152 | (20) | 107 | (70) | 0.91 | (0.57–1.47) | 116 | (76) | 0.78 | (0.47–1.32) |
| Communication | ||||||||||
| Received info from | ||||||||||
| Neither AAFP nor AAP | 330 | (43) | 195 | (59) | 1 | 218 | (66) | 1 | ||
| AAFP only | 135 | (17) | 97 | (72) | 1.77 | (1.14–2.73)* | 108 | (80) | 2.06 | (1.27–3.32)** |
| AAP only | 268 | (35) | 236 | (88) | 5.11 | (3.32–7.85)** | 250 | (93) | 7.14 | (4.20–12.12)** |
| Both | 43 | (6) | 25 | (58) | 0.96 | (0.50–1.83) | 33 | (77) | 1.70 | (0.81–3.57) |
Note. Items assessing knowledge of professional organizations’ positions specified AAFP for family physicians and AAP for pediatricians. AAFP: American Academy of Family Physicians. AAP: American Academy of Pediatrics. HPV: human papillomavirus. OR: odds ratio. CI: confidence interval. VFC: Vaccines for Children.
p < 0.05
p < 0.01
Organizational Reach
Overall, 47% of family physicians reported receiving information from AAFP, and 62% of pediatricians reported receiving information from AAP (Figure 1). Other information sources were drug companies (52%), the CDC (31%), insurance companies (9%), and the President’s Cancer Panel (2%). Among physicians who received any information, 82% heard from at least one non-profit source (i.e., AAP, CDC, AAFP, or the President’s Cancer Panel). Twenty-four percent of physicians reported not receiving information from any of the sources listed.
Figure 1.
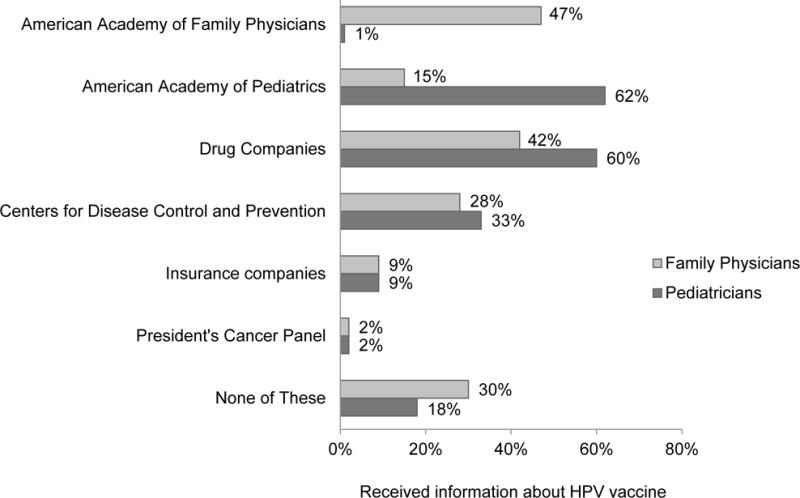
Sources of direct-to-physician communication about HPV vaccine.
Content and Perceived Influence of Organizational Communication
Among physicians who reported receiving information from professional organizations, most reported hearing from AAFP or AAP that they should give strong recommendations for HPV vaccine to all 11- to 12-year-old males and females (Table 2). At least half (53%) of physicians who reported receiving information from AAFP (53%) or AAP (72%) heard that HPV vaccination rates are too low. Smaller proportions heard that it is important to identify parents’ concerns about HPV vaccination and few physicians reported hearing that recommendations should be based on adolescents’ risks of getting HPV. A number of physicians reached by AAFP or AAP indicated that the information made them discuss HPV vaccination more often (36% and 26%, respectively), recommend it more often (35% and 25%), or recommend it more strongly (27% and 29%). Several physicians reached by AAFP (31%) or AAP (47%) reported that the information had no effect.
Table 2.
Content and influence of professional organizations’ communication about HPV vaccine.
| Physicians who received information from: | ||||
|---|---|---|---|---|
| American Academy of Family Physicians (n=178) | American Academy of Pediatrics (n=311) | |||
|
| ||||
| n | (%) | n | (%) | |
| Content | ||||
| In the last year, I have heard from [AAFP/AAP] that… | ||||
| I should give a strong recommendation to all 11–12 year old boys | 115 | (65) | 233 | (75) |
| I should give a strong recommendation to all 11–12 year old girls | 134 | (75) | 252 | (81) |
| HPV vaccination rates are too low | 95 | (53) | 223 | (72) |
| It is important to identify parents’ concerns | 64 | (36) | 107 | (34) |
| Recommendations should be based on adolescents’ risk of getting HPV | 29 | (16) | 24 | (8) |
| Influence | ||||
| Information from [AAFP/AAP] about HPV vaccine for 11–12 year olds made me… | ||||
| Discuss it more often | 64 | (36) | 81 | (26) |
| Recommend it more often | 63 | (35) | 78 | (25) |
| Recommend it more strongly | 48 | (27) | 90 | (29) |
| Had no effect | 56 | (31) | 146 | (47) |
Note. AAFP: American Academy of Family Physicians. AAP: American Academy of Pediatrics. HPV: human papillomavirus. Content and influence assessed among physicians who reported receiving information from AAP (n=311) or AAFP (n=178).
Knowledge of Professional Organizations’ Position on HPV Vaccination
When asked to identify their professional organizations’ positions on HPV vaccination for boys, 56% of family physicians and 85% of pediatricians knew that AAFP or AAP recommended routine vaccination at ages 11 and 12. Few physicians incorrectly reported that the recommendation for boys was to give HPV vaccine at the physician’s discretion (14% and 5%) or that professional organizations had no position (<1% and <1%), or reported being unsure of the position (29% and 9%). For girls, 66% of family physicians and 90% of pediatricians knew that AAFP or AAP recommended routine vaccination at ages 11 and 12. Few reported incorrectly that the recommendation for girls was to give HPV vaccines at the physician’s discretion (8% and 3%) or that professional organizations had no position (1% and <1%), or reported being unsure of the position (25% and 7%).
Bivariate analyses suggested several correlates of knowing the position for boys and girls (Table 1), yet these variables did not retain significance in multivariable models. In multivariable analyses, knowing AAFP or AAP’s position on HPV vaccination for boys was less common for family physicians versus pediatricians (OR=0.30 [95% CI 0.18, 0.48]), and was more common for physicians who provided 10% to 49% versus <10% of their HPV vaccine doses through the VFC program (OR=1.72 [95% CI 1.13, 2.63]). Knowing the position was also independently associated with receiving information from AAFP (OR=3.24 [95% CI 2.00, 5.25]) or AAP (OR=2.46 [95% CI 1.50, 4.05]) versus neither source. Knowing AAFP or AAP’s position on HPV vaccination for girls was less common for family physicians versus pediatricians (OR=0.28 [95% CI 0.16, 0.46]) and was more common for physicians who provided 10% to 49% versus <10% of their HPV vaccines through the VFC program (OR=1.73 [95% CI 1.08, 2.77]). Knowing the position was also independently associated with receiving information from AAFP (OR=3.52 [95% CI 2.11, 5.91]) or AAP (OR=3.45 [95% CI 1.90, 6.27]) versus neither source.
HPV Vaccine Recommendation Quality
Physicians who reported receiving information from AAFP (among family physicians) or AAP (among pediatricians) were not more likely to have high HPV vaccine recommendation quality scores than those who did not receive information from these sources (Figure 2 Panel A). Physicians who reported that they had versus had not heard from AAFP or AAP to give strong recommendations were more likely to have high recommendation quality scores for boys (57% vs. 24%; χ2 = 39.87; p<0.001) and for girls (54% vs. 27%; χ2 = 21.81; p<0.001; Figure 2 Panel B). Similarly, physicians who reported that they did versus did not know their professional organization’s positions on HPV vaccination were more likely to have high recommendation quality scores for boys (54% vs. 26%; χ2 = 48.14; p<0.001) and for girls (52% vs. 25%; χ2 = 37.26; p<0.001; Figure 2 Panel C).
Figure 2.
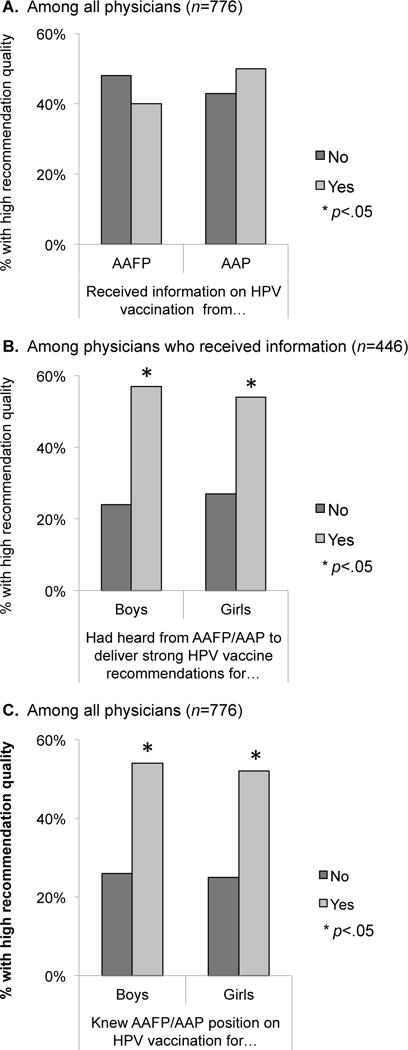
Associations between professional organizations’ communication and HPV vaccine recommendation quality.
DISCUSSION
In this survey of family physicians and pediatricians, we found that professional organizations’ communication about HPV vaccination was positively associated with physicians’ knowledge and recommendation practices. Most physicians reached by AAFP or AAP reported receiving the message that they should deliver strong recommendations for HPV vaccination to 11- and 12-year-old patients. Similarly, physicians reached by professional organizations had higher odds of knowing their professional organizations’ position for routine HPV vaccination of patients in this age range. Although receiving information was not associated with using higher-quality communication practices to recommend HPV vaccination, receiving the message to deliver strong recommendations and knowing professional organizations’ position were associated with recommendation quality. Our findings support previous studies about the importance of HPV endorsement by professional organizations on physicians’ recommendation practices.22,23 Importantly, our findings suggest that professional organizations’ communications about HPV vaccination may play a role in influencing physicians’ knowledge and recommendation practices related to HPV vaccination.
Our findings also show that the reach of these organizations may be somewhat limited. Fewer than half of family physicians reported receiving information from AAFP, and less than two-thirds of pediatricians reported receiving information from AAP. Family physicians indicated that they were less likely than pediatricians to know their professional organization’s positions on HPV vaccination. However, among family physicians who reported receiving information, most (69%) said they improved their recommendation practices by discussing HPV vaccination more often, recommending it more often, or recommending it more strongly, as a result. Increasing the intensity of professional organizations’ quality improvement efforts, particularly for family physicians, is likely needed to extend their reach, clarify their positions on HPV vaccination, and maximize their influence on physicians’ recommendation practices.
Professional organizations have recently launched initiatives to accomplish this goal. Most notably, AAP partnered with the CDC to develop practice tools and provide outreach and training to physician’s offices.24 Products of this collaboration include the HPV vaccination “Champion Toolkit,” which emphasizes the need for providers to recommend vaccination for all boys and girls at ages 11–12.25 Recently developed materials also include “Provider Resources for Vaccine Conversations with Parents,” created by the CDC, AAFP and AAP to help providers communicate more effectively with parents about vaccinating their children.26 Since many physicians in our study reported improving their HPV vaccine recommendation practices as a result of information they received from AAFP or AAP, these recent efforts may help to improve HPV vaccination coverage. However, given the importance of communicating the need for strong recommendations, further research that experimentally tests these messages on physician behaviors is needed to more fully understand their impact.
Our findings also suggest that care is needed to craft messages that effectively communicate professional organizations’ positions for routine HPV vaccination. Although physicians who reported being reached by AAFP or AAP were more likely to know their professional organizations’ position, the correlation was not perfect; for example, 28% of physicians reached by AAFP did not know the position for boys. While physicians may be receiving the information, the purpose of these messages is not being effectively conveyed. Ineffective messaging may explain why receiving information was not associated with higher-quality HPV vaccine recommendation practices. Future research could support professional organizations’ quality improvement efforts by developing and testing messages that effectively communicate the goal of routine and timely HPV vaccine delivery.
Our study is among the first to evaluate HPV vaccine-related communications from professional organizations, which prior research suggests are influential for informing primary care physicians’ vaccine recommendation practices.21,22 Despite barriers to HPV vaccination, a systematic review found that physician recommendations were a primary predictor of successful vaccination uptake.27 Limitations with our study should be considered. Given of our cross-sectional design, we cannot draw causal inferences regarding the associations between professional organizations’ communications and physicians’ knowledge and recommendation practices. Our self-reported data are similarly subject to social-desirability bias, which may have led physicians to overestimate the quality of their HPV vaccination recommendation practices. A modest response rate (33%) is another limitation, especially given that respondents previously agreed to be on the survey panel, although physician surveys are often subject to challenges with response rates.28,29 Furthermore, because our sample came from a standing national panel, our findings may reflect the views of physicians who are more likely to participate in research activities, and may not generalize to other physicians. We also acknowledge that we did not have information on length or frequency of messaging, precluding an assessment of dose effect. Nevertheless, we collected information on type of content physicians’ received from professional organizations, which allowed us to explore associations between content and physician practice. Messages communicated by professional organizations are likely similar in terms of content, which may have led to some degree of misclassification in physicians’ reports of message source. Finally, future research is needed to assess the influence of other important information sources about HPV vaccine, including CDC and drug companies.
Conclusion
Organizational change theory suggests that disseminating new tools and practices is often heavily dependent on opinion leaders,30 and in the case of HPV vaccination, these leaders appear to include professional organizations like AAFP and AAP. Our study suggests that professional organizations’ messages about HPV vaccination were associated with physicians’ knowledge and recommendation practices, and that physicians perceived these communications as influential. However, several physicians reported not being reached by a professional organization and a substantial minority did not know their organization’s position for routine HPV vaccination of boys and girls. Although there are many steps between intent and delivery of HPV vaccination, our study suggests that forthcoming initiatives by professional organizations that address these informational needs may make a difference in HPV vaccination acceptance through physician recommendations. It is noteworthy that one of our main findings suggests that physicians who received or did not receive information from AAFP or AAP reported no differences in HPV vaccine recommendation quality. Therefore, to ensure that all physicians are receiving accurate messaging about HPV vaccination recommendations, future research should seek to evaluate these efforts to better understand the role that professional organizations play in disseminating practice guidelines.
Acknowledgments
Sources of funding: The funder played no role in the study design, planning, implementation, analysis, or reporting of the findings, or decision to publish. Dr. Brewer has also received vaccine-related grants and honoraria from or served on paid advisory boards for Merck Sharp & Dohme Corp and GlaxoSmithKline. A research grant from Pfizer supported this study (Dr. Brewer). Additional support was received from the Cancer Control Education Program at UNC Lineberger Comprehensive Cancer Center (R25 CA57726) and a career development award from the National Cancer Institute (K22 CA186979).
Footnotes
Conflicts of interest: The remaining authors (YH, MG, BR) have no conflicts to disclose.
References
- 1.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sexually transmitted diseases. 2013;40(3):187–193. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 2.Dunne EF, Markowitz LE, Saraiya M, et al. CDC grand rounds: reducing the burden of HPV-associated cancer and disease. MMWR Morbidity and Mortality Weekly Report. 2014;63(4):69–72. [PMC free article] [PubMed] [Google Scholar]
- 3.Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years—United States, 2015. MMWR.Morbidity and Mortality Weekly Report. 2016;65(33):850–858. doi: 10.15585/mmwr.mm6533a4. [DOI] [PubMed] [Google Scholar]
- 4.Markowitz LE, Tsu V, Deeks SL, et al. Human papillomavirus vaccine introduction–the first five years. Vaccine. 2012;30(Supp 5):F139–F148. doi: 10.1016/j.vaccine.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 5.Chesson HW, Ekwueme DU, Saraiya M, Dunne EF, Markowitz LE. The Estimated Impact of Human Papillomavirus Vaccine Coverage on the Lifetime Cervical Cancer Burden Among Girls Currently Aged 12 Years and Younger in the United States. Sexually transmitted diseases. 2014;41(11):656–659. doi: 10.1097/OLQ.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel PR, Berenson AB. Sources of HPV vaccine hesitancy in parents. Human vaccines & immunotherapeutics. 2013;9(12):2649–2653. doi: 10.4161/hv.26224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilkey MB, Moss JL, McRee A-L, Brewer NT. Do correlates of HPV vaccine initiation differ between adolescent boys and girls? Vaccine. 2012;30(41):5928–5934. doi: 10.1016/j.vaccine.2012.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stokley S, Jeyarajah J, Yankey D, et al. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014-United States. MMWR.Morbidity and mortality weekly report. 2014;63(29):620–624. [PMC free article] [PubMed] [Google Scholar]
- 9.Reiter PL, Gilkey MB, Brewer NT. HPV vaccination among adolescent males: results from the National Immunization Survey-Teen. Vaccine. 2013;31(26):2816–2821. doi: 10.1016/j.vaccine.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brewer NT, Gottlieb SL, Reiter PL, et al. Longitudinal predictors of HPV vaccine initiation among adolescent girls in a high-risk geographic area. Sexually transmitted diseases. 2011;38(3):197–204. doi: 10.1097/OLQ.0b013e3181f12dbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vadaparampil ST, Kahn JA, Salmon D, et al. Missed clinical opportunities: provider recommendations for HPV vaccination for 11–12 year old girls are limited. Vaccine. 2011;29(47):8634–8641. doi: 10.1016/j.vaccine.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sussman AL, Helitzer D, Bennett A, Solares A, Lanoue M, Getrich CM. Catching Up With the HPV Vaccine: Challenges and Opportunities in Primary Care. The Annals of Family Medicine. 2015;13(4):354–360. doi: 10.1370/afm.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulczycki A, Qu H, Shewchuk R. Primary Care Physicians’ Adherence to Guidelines and Their Likelihood to Prescribe the Human Papillomavirus Vaccine for 11-and 12-Year-Old Girls. Women’s Health Issues. 2015 doi: 10.1016/j.whi.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilkey MB, McRee A-L. Provider Communication about HPV Vaccination: A Systematic Review. Human vaccines & immunotherapeutics. 2016;12(6):1454–1468. doi: 10.1080/21645515.2015.1129090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Academy of Pediatrics. HPV vaccine recommendation letter. 2014 http://www.immunize.org/letter/recommend_hpv_vaccination.pdf. Accessed December 15 2015.
- 16.Gilkey MB, Malo TL, Shah PD, Hall ME, Brewer NT. Quality of physician communication about human papillomavirus vaccine: Findings from a national survey. Cancer Epidemiology Biomarkers & Prevention. 2015;24(11):1673–1679. doi: 10.1158/1055-9965.EPI-15-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GfK Knowledge Networks. Physicians Consulting Network. 2015 http://www.knowledgenetworks.com/resources/pcn.html. Accessed August 15 2015.
- 18.Gilkey MB, Moss JL, Coyne-Beasley T, Hall ME, Shah PD, Brewer NT. Physician communication about adolescent vaccination: How is human papillomavirus vaccine different? Preventive medicine. 2015;77:181–185. doi: 10.1016/j.ypmed.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Vaccines for Children Program (VFC) 2015 http://www.cdc.gov/vaccines/programs/vfc/index.html. Accessed August 15 2015.
- 20.US Census Bureau. Geographic terms and concepts - census divisions and census regions. 2015 https://www.census.gov/geo/reference/gtc/gtc_census_divreg.html. Accessed August 15 2015.
- 21.Daley MF, Liddon N, Crane LA, et al. A national survey of pediatrician knowledge and attitudes regarding human papillomavirus vaccination. Pediatrics. 2006;118(6):2280–2289. doi: 10.1542/peds.2006-1946. [DOI] [PubMed] [Google Scholar]
- 22.Riedesel JM, Rosenthal SL, Zimet GD, et al. Attitudes about human papillomavirus vaccine among family physicians. Journal of pediatric and adolescent gynecology. 2005;18(6):391–398. doi: 10.1016/j.jpag.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Zimet GD. Improving adolescent health: focus on HPV vaccine acceptance. Journal of Adolescent Health. 2005;37(6):S17–S23. doi: 10.1016/j.jadohealth.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 24.American Academy of Pediatrics. AAP news: grant will support QI methods to improve HPV vaccination. 2014 http://www.aappublications.org/content/35/11/26.2. Accessed August 15 2015.
- 25.Amercian Academy of Pediatrics. HPV champion toolkit: HPV vaccine is cancer prevention. 2015 https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Pages/HPV-Champion-Toolkit.aspx. Accessed December 11 2015.
- 26.Centers for Disease Control and Prevention. Provider resources for vaccine conversations with parents. 2015 http://www.cdc.gov/vaccines/hcp/conversations/index.html. Accessed December 11 2015.
- 27.Brewer NT, Fazekas KI. Predictors of HPV vaccine acceptability: a theory-informed, systematic review. Preventive medicine. 2007;45(2):107–114. doi: 10.1016/j.ypmed.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Kellerman SE, Herold J. Physician response to surveys: A review of the literature. American journal of preventive medicine. 2001;20(1):61–67. doi: 10.1016/s0749-3797(00)00258-0. [DOI] [PubMed] [Google Scholar]
- 29.Flanigan TS, McFarlane E, Cook S. Conducting survey research among physicians and other medical professionals: a review of current literature. Paper presented at: Proceedings of the Survey Research Methods Section, American Statistical Association. 2008 [Google Scholar]
- 30.Weick KE, Quinn RE. Organizational change and development. Annual review of psychology. 1999;50(1):361–386. doi: 10.1146/annurev.psych.50.1.361. [DOI] [PubMed] [Google Scholar]


