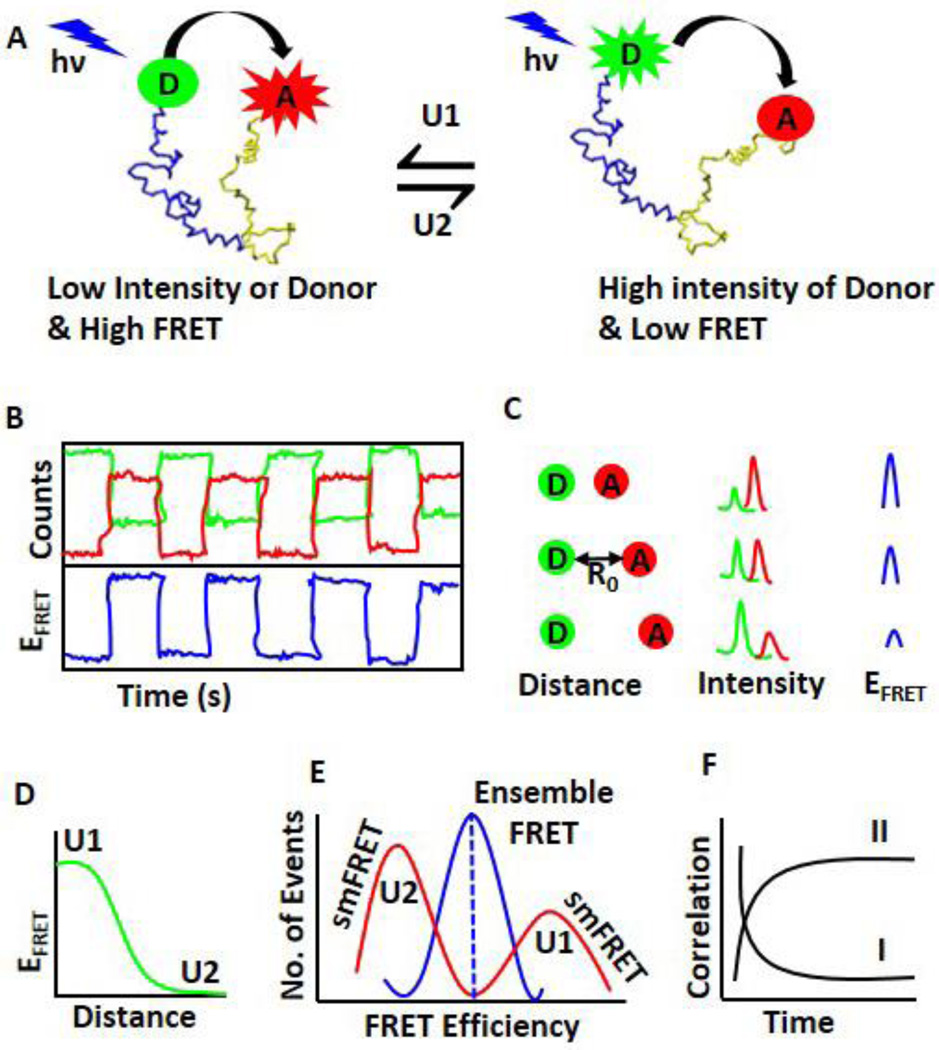
Fig. 2.
smFRET to study conformational dynamics of individual molecules. A. A representative molecular conformation change and corresponding FRET efficiency. A donor (D, green) and an acceptor (A, red) are covalently attached at two ends of a biomolecule. In one conformation seen on the left (U1), the distance between the donor and the acceptor is very short and thus the FRET efficiency is high (Equation 1). Experimentally, low intensity of the donor and very high intensity of the acceptor are observed. For the conformer on the right (U2), the distance between the donor and the acceptor is large, and consequently the FRET efficiency is very low. The intensity of the donor will be high and the intensity of the acceptor will be low. B. A representative diagram of the intensity fluctuations (top panel) of the donor (green), acceptor (red), and corresponding FRET efficiency (blue, bottom panel). C. The distance between the donor and the acceptor is crucial for efficient FRET (Equation 1). The distance (left column) and its corresponding donor and the acceptor intensities (middle column) as well as FRET efficiency (right column) are shown here. The distance at which the energy transfer efficiency is 50% called as Förster distance (R0). D. The relationship between FRET efficiency and D-A distance. The high FRET efficiency corresponds to the conformer U1, and the low FRET efficiency corresponds to the conformer U2. E. Difference between smFRET and ensemble FRET. smFRET can distinguish two different conformers using FRET efficiency, but ensemble FRET can only give an average value. F. The dynamics is determined by the statistical analysis of autocorrelation (I) and cross-correlation (II) of time-trace of fluctuations of the donor and the acceptor.