Figure 9.
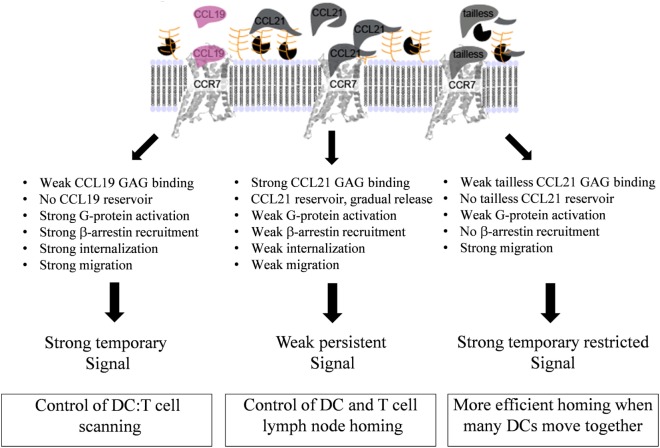
Model for diverse mechanisms of CCL19, CCL21, and tailless-CCL21 in regulation of CCR7 activation. As revealed by the structure comparison (Figure 5), CCL21 harbors structural motifs indicating a unique ability to strongly interact with GAGs, also supported by earlier reports. Increased GAG binding builds a local CCL21 reservoir at the cell surface, with CCL21 present in an inhibited, non-inhibited, or even facilitated form dependent on the GAG carrying it. In contrast, CCL19 only displays weak GAG interaction and readily diffuses away from the cell if not immediately bound to CCR7. GAG-bound CCL21 on the contrary, may either (i) interact directly with CCR7 if bound to polysialic acid, (ii) interact with CCR7 after GAG detachment caused by protease induced tail removal, with tailless-CCL21 probably being immediately ready for receptor engagement since release is expected to occur at the DC surface and thus in close proximity to CCR7 molecules ready to capture the chemokine, or (iii) interact with CCR7 after transfer from a GAG that presents CCL21 in its inhibited state (CS-B), to a GAG that allows CCR7 engagement (polysialic acid), with CS-B here acting as a dormant reservoir. CCL19 does not form a local reservoir, and once bound to CCR7, it gets quickly internalized and degraded, which is not the case for CCL21. Our model thus predicts that CCL19 and CCL21 induce differential CCR7 activation, with CCL19 creating a short-lived (temporary) signal, and CCL21 displaying a weaker, but more persistent CCR7 activation profile.