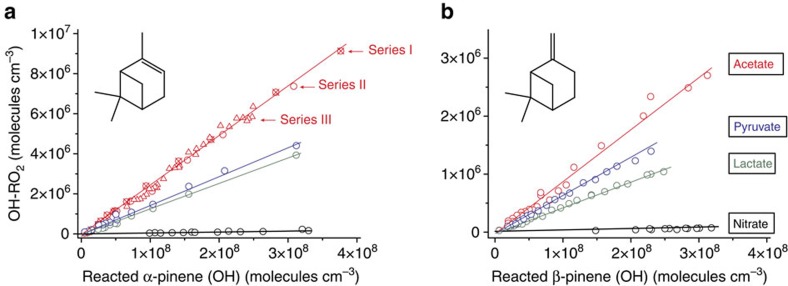
Figure 3. Estimated RO2 radical concentrations as a function of reacted α- and β-pinene.
The given concentrations for the different reagent ions were obtained using a calibration factor from absolute sulphuric acid calibration that is in line with the calculated, lower end calibration factor of an ion-molecule reaction. Reagent ions (colour coding): nitrate (black), lactate (green), pyruvate (blue) and acetate (red). The reaction time in all experiments was 7.9 s. (a) α-Pinene reaction: series I and II from acetate ionization and the results from the other reagent ions were obtained from α-pinene ozonolysis with OH radical formation via O3+α-pinene. [O3]=6.1 × 1011 and [α-pinene]=(1.2–81) × 1010 molecules cm−3. Series III (acetate ionization) shows findings from combined TME/α-pinene ozonolysis. O3+TME generates additional OH radicals. [O3]=9.1 × 1011, [TME]=(1.8–110) × 109, [α-pinene]=1.0 × 1011 molecules cm−3. (b) β-Pinene reaction: combined TME/β-pinene ozonolysis with preferred OH radical formation via the O3+TME reaction, [O3]=9.1 × 1011, [TME]=(1.8–95) × 109, [β-pinene]=1.05 × 1011 molecules cm−3.