Latent Toxoplasma infection prevalence among HIV-infected patients was 11.4% and was associated with worse brain function. The mechanism may involve damage from improved immune responses to subclinical reactivation of Toxoplasma. Longitudinal and intervention studies should be pursued.
Keywords: latent Toxoplasma infection, HIV-1 infection, Anti–Toxoplasma gondii IgG, latent toxoplasmosis, neurocognitive impairment
Abstract
Background. Human immunodeficiency virus (HIV)–associated neurocognitive disorders persist despite suppressive antiretroviral therapy (ART). Because latent Toxoplasma infection (LTI) may adversely impact brain function, we investigated its impact on neurocognitive impairment (NCI) in people living with HIV disease.
Methods. Two hundred sixty-three HIV-infected adults underwent comprehensive neurocognitive assessments and had anti-Toxoplasma gondii immunoglobulin G (anti-Toxo IgG) measured by qualitative and quantitative enzyme-linked immunosorbent assays.
Results. Participants were mostly middle-aged white men who were taking ART (70%). LTI was detected in 30 (11.4%) participants and was associated with a significantly greater prevalence of global NCI (LTI positive [LTI+] = 57% and LTI negative [LTI–] = 34%) (odds ratio, 1.67; 95% confidence interval, 1.17–2.40; P = .017). Deficits were more prevalent in the LTI+ vs the LTI– group in 6 of 7 cognitive domains with statistical significance reached for delayed recall (P < .01). The probability of NCI increased with higher CD4+ T-cell counts among LTI+ individuals but with lower CD4+ T-cell counts in LTI– persons. A strong correlation (r = .93) between anti-Toxo IgG levels and global deficit score was found in a subgroup of 9 patients. Biomarkers indicative of central nervous system inflammation did not differ between LTI+ and LTI– participants.
Conclusions. In this cross-sectional analysis, LTI was associated with NCI, especially in those with higher CD4+ T-cell counts. Longitudinal studies to investigate the role of neuroinflammation and neuronal injury in LTI patients with NCI and trials of anti-Toxoplasma therapy should be pursued.
Human immunodeficiency virus (HIV)–associated neurocognitive disorder (HAND) is a well-recognized complication of HIV-1 infection that can impede employment, activities of daily living, and ultimately survival [1]. Antiretroviral therapy (ART) alone is often not sufficient to restore full cognitive functioning, suggesting that the cause of persisting neurocognitive impairment (NCI) may not be fully understood. Chronic coinfections, such as cytomegalovirus [2] or hepatitis C virus [3], are associated with NCI and may contribute to persistent NCI during ART.
Another chronic coinfection, Toxoplasma gondii, infects up to one-third of the world's population [4]. Toxoplasma gondii is an obligate intracellular protozoal parasite and humans acquire infection after ingesting the cysts in undercooked meat or contaminated fruits or vegetables. The rapidly replicating tachyzoite form disseminates throughout the body before transforming into the slowly replicating bradyzoites within cysts found mainly in the brain and skeletal muscle [5]. Latent Toxoplasma infection (LTI) produces no symptoms. Waning cell-mediated immunity, as in AIDS, can result in reactivation of LTI and cause encephalitis [6].
Recent evidence from animal and human studies suggests that LTI can result in behavioral changes, including increased impulsivity, aggression, and suicide attempts [7, 8]; difficulties with learning in mice [9]; and with memory [10], reaction time [11], and higher risk of traffic accidents in humans [12]. Whether LTI contributes to NCI in HIV-infected adults in the absence of clinical encephalitis is unknown. To address this, we analyzed the associations between LTI and (i) NCI; (ii) anti-Toxoplasma immunoglobulin G (anti-Toxo IgG) levels; and (iii) cerebrospinal fluid (CSF) inflammatory biomarkers.
METHODS
Participants
We identified 263 HIV-infected participants, enrolled in several National Institutes of Health–funded cohort studies at the University of California, San Diego (UCSD) HIV Neurobehavioral Research Program, all of whom had undergone comprehensive neurocognitive assessments and had anti-Toxo IgG measured by qualitative assay for routine screening in HIV clinical care at the UCSD Owen Clinic. Toxoplasma encephalitis presents as an acute illness with new-onset seizures, hemiparesis, or other acute focal neurological signs [13]. Participants in the analysis described here were ambulatory patients without acute illness or focal neurologic disturbances on medical examination. All protocols were approved by the UCSD Human Research Protections Program, and all subjects provided written informed consent.
Neurocognitive Functioning Assessments
All participants were tested using a comprehensive neurocognitive test battery to assess 7 cognitive domains as previously described [14]: learning, recall, attention/working memory, speed of information processing, verbal fluency, executive functions, and motor skills. Individual test scores were standardized using published, normative data that adjust for age, education, sex, and ethnicity and were combined to create global- and domain-specific deficit scores (GDS and DDS, respectively) that range from 0 (normal) to 5 (severely impaired) [15]. The GDS is an automated method to detect impairment, requires lower-than-expected performance in several domains, and ignores higher-than-expected performance. Consistent with published, well-validated procedures, global NCI was defined as a GDS ≥ 0.5 and domain-specific NCI was defined as DDS > 0.5.
Laboratory Assays
Blood and, in subjects who consented to lumbar puncture, CSF specimens were collected at the time of neurocognitive function testing and stored at −80°C. LTI diagnosis was defined by qualitative detection of anti-Toxo IgG (TX022G assay, Calbiotech, Spring Valley, California) with a manufacturer-specified qualitative cutoff of 1.1. Levels of anti-Toxo IgG in IgG-positive participants were estimated based on the colorimetric signal intensity per the manufacturer's instructions. Soluble biomarkers in CSF were measured by bead suspension arrays (Millipore, Billerica, Massachusetts) on a BioPlex 100 platform (Bio-Rad, Hercules, California) for monocyte chemoattractant protein 1 (MCP-1) and interferon γ–induced protein 10 (IP-10), and enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minnesota) for soluble CD14 (sCD14) and neopterin. HIV RNA levels were quantified in plasma and CSF by reverse transcription–polymerase chain reaction (Roche Amplicor, version 1.5, lower limit of quantitation 50 copies/mL). CD4+ T-cell counts were measured in blood by flow cytometry.
Statistical Analysis
Differences between LTI-positive (LTI+) and LTI-negative (LTI–) participants and between trimethoprim-sulfamethoxazole (TMP-SMX) users and nonusers were compared using t tests or Wilcoxon rank-sum tests for means and medians, or χ2 or Fisher exact tests for proportions. Demographic, disease, and treatment variables were screened by univariable logistic regression to estimate NCI at a 15% significance level. Variables below this screening level were combined with LTI status and their interaction in a multivariable logistic model and tested. The correlation of anti-Toxo IgG with each of CD4+ T-cell count, GDS, and DDS was estimated using Pearson product moment or Spearman correlation coefficients. For all testing, required parametric assumptions were verified, and data transformations or nonparametric methods were applied when needed. Two-tailed tests and a 5% significance level were used for hypothesis testing unless indicated otherwise. Because adjustments were not made for multiple comparisons, the statistical significance of our findings must be interpreted accordingly.
RESULTS
Demographic and Disease Characteristics
Demographic, disease, and treatment characteristics are shown in Table 1. Participants had a mean age of 42 years (SD, 9.3 years), a mean education of 12.6 years (SD, 2.4 years), and were predominantly white (59%), male (90%), and taking ART (70%). Less than half of patients had undetectable levels of HIV RNA in blood, but the percentage was higher in LTI+ than LTI– participants (57% vs 44%; P = .18). LTI was detected in 30 (11.4%) participants. No differences in race/ethnicity were seen between LTI– and LTI+ individuals (P > .8). The median current CD4+ T-cell count was 387 cells/µL (interquartile range, 231–582 cells/µL). Neither CD4+ T-cell counts nor CD4+ T-cell nadir was significantly associated with LTI status (CD4+ T-cell count: P = .98; CD4+ nadir: P = .91). Overall, LTI+ and LTI– participants had similar demographics and HIV disease characteristics.
Table 1.
Demographic, Disease, and Treatment Characteristics of Subjects With and Without Latent Toxoplasma gondii Infection
| Characteristic | LTI– (n = 233) | LTI+ (n = 30) | P Value |
|---|---|---|---|
| Age, y, mean (SD) | 41.8 (9.1) | 44.6 (10.4) | .12 |
| Education, y, mean (SD) | 12.6 (2.4) | 12.4 (2.9) | .82 |
| Male sex, No. (%) | 211 (91) | 26 (87) | .51 |
| Ethnicity, No. (%) | .81 | ||
| Black | 36 (15) | 5 (17) | |
| Hispanic | 49 (21) | 7 (23) | |
| White | 138 (59) | 16 (53) | |
| Other | 10 (4) | 2 (7) | |
| HIV RNA in plasma, log10 copies/mLa, mean (SD) | 3.0 (1.4) | 2.7 (1.3) | .34 |
| Undetectablea, No. (%) | 94 (44) | 17 (57) | .18 |
| ART useb, No. (%) | 161 (69) | 22 (76) | .47 |
| On ART and RNA undetectablec, No. (%) | 92 (43) | 17 (59) | .11 |
| CD4+ count, cells/µLd, median (IQR) | 395 (214–583) | 330 (238–535) | .98 |
| CD4+ nadir, cells/µLe, median (IQR) | 211 (63–280) | 195 (176–281) | .91 |
| GDS ≥ 0.5, No. (%) | 79 (34) | 17 (57) | .017 |
Abbreviations: –, negative; +, positive; ART, antiretroviral therapy; GDS, global deficit score; HIV, human immunodeficiency virus; IQR, interquartile range; LTI, latent Toxoplasma infection; SD, standard deviation.
a n = 216 for LTI–.
b n = 232 for LTI– and n = 29 for LTI+.
c n = 215 for LTI– and n = 29 for LTI+.
d n = 221 for LTI– and n = 29 for LTI+.
e n = 105 for LTI– and n = 12 for LTI+.
Neurocognitive Impairment by LTI Status
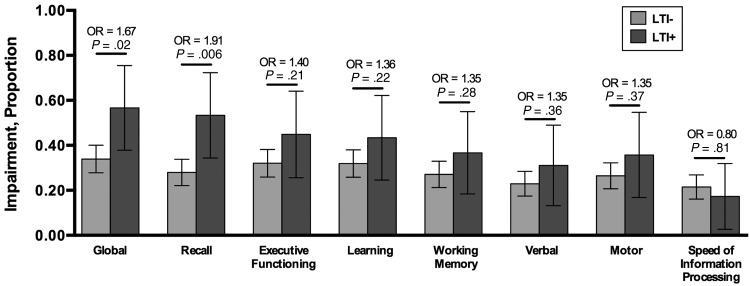
Figure 1 displays global and domain impairment by LTI status. LTI was associated with a significantly greater prevalence of global NCI (LTI+ = 57% and LTI– = 34%; odds ratio [OR], 1.67; 95% confidence interval [CI], 1.17–2.40; P = .017). Prevalence of impairment in the domain of delayed recall also differed strikingly (LTI+ = 53% and LTI– = 28%; OR, 0.006; P = .006). For 6 of 7 cognitive domains, deficits were more prevalent in the LTI+ than the LTI– group.
Figure 1.
Global and domain impairment by latent Toxoplasma infection (LTI) status. Deficits were more prevalent in the LTI-positive (LTI+) vs the LTI-negative (LTI–) group in 6 of 7 cognitive domains, and these differences reach statistical significance for global impairment and delayed recall. Abbreviation: OR, odds ratio.
Probability of NCI by LTI Status and CD4+ T-Cell Counts
LTI+ and LTI– patients had similar mean current or nadir CD4+ T-cell counts. CD4+ T-cell count was significantly related to NCI (P < .001) univariably and, in a multivariable logistic regression model of risk of NCI (overall P < .001; Figure 2), the probability of NCI increased with increasing CD4+ T-cell counts in LTI+ patients, whereas NCI was inversely related to CD4+ T-cell counts among those without LTI (LTI− P = .021, CD4+ P = .001; and their interaction P = .019). CD4+ T-cell count was the only demographic, disease, or treatment variable significantly correlated to NCI in univariate analysis.
Figure 2.
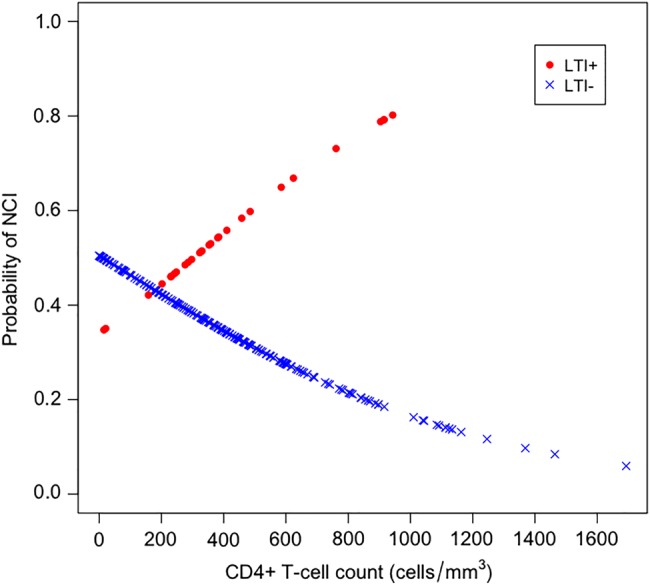
Logistic regression model of the probability of neurocognitive impairment (NCI) by latent Toxoplasma infection (LTI) status and CD4+ T-cell counts. While risk of NCI decreases with higher CD4+ T-cell counts in LTI-negative (LTI–) persons (blue crosses), the opposite effect is found in LTI-positive (LTI+) persons (red dots). At CD4+ T-cell counts near 200 cells/µL, the levels of risk converge.
Neurocognitive Functioning and CD4+ T-Cell Count Correlation With Anti-Toxo IgG Levels
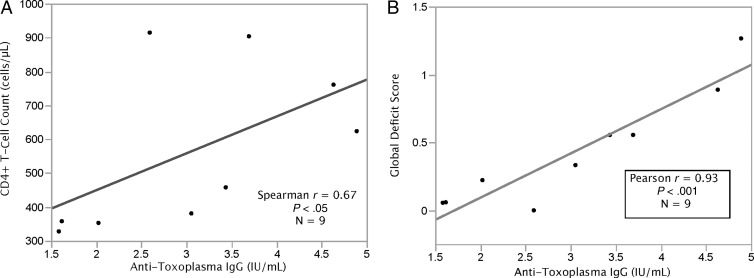
In a small subgroup of anti-Toxo IgG–positive participants with stored serum specimens who underwent quantitative anti-Toxo IgG testing (n = 9), higher anti-Toxo IgG levels correlated with higher CD4+ T-cell counts (r = 0.67, P < .05; Figure 3A). Anti-Toxo IgG levels and GDS were highly correlated (r = 0.93, P < .001, Figure 3B). In this subgroup, higher anti-Toxo IgG levels were also correlated with worse scores in 3 of 7 cognitive domains: (i) delayed recall (r = 0.82, P = .007), (ii) learning (r = 0.70, P = .035), and (iii) motor ability (r = 0.81, P = .009).
Figure 3.
CD4 counts and global deficit scores correlate with anti-Toxoplasma gondii immunoglobulin G (IgG) levels. A, Higher anti-Toxoplasma IgG levels correlated with higher CD4+ T-cell counts. B, Anti-Toxoplasma IgG levels were highly correlated with global deficit score.
LTI and CSF Biomarkers
To examine a possible role for central nervous system (CNS) inflammation in the pathogenesis of these relationships, we studied 58 participants (5 LTI+ and 53 LTI–) who previously had 4 biomarkers of inflammation (MCP-1, IP-10, sCD14, and neopterin) measured in CSF. These biomarkers have previously been associated with CNS inflammation or cognitive impairment in HIV infection [16]. None of the biomarkers differed between LTI+ and LTI– participants (all t test P values >.2).
TMP-SMX and Neurocognitive Performance Among LTI+ Participants
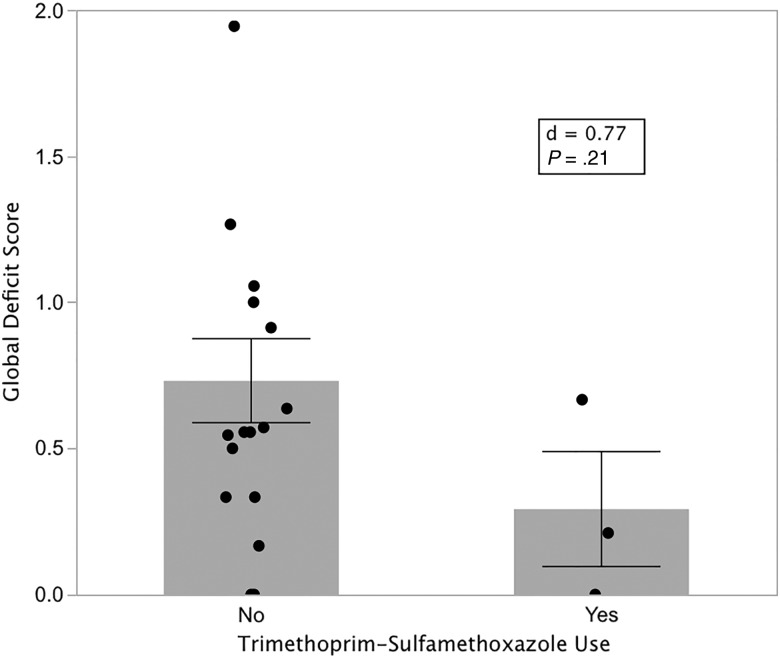
Because of its activity against Toxoplasma and use in prevention of HIV-related clinical Toxoplasma infections, we examined the impact of current TMP-SMX use on neurocognitive performance among the 21 of 30 LTI+ participants for whom data were available. Because this cohort was predominately immune-reconstituted on ART and not at high risk of reactivation of Toxoplasma or other opportunistic infections, only 4 participants reported TMP-SMX use. One of these participants was excluded for hepatitis C virus seropositivity. Although not statistically significant, the remaining 3 TMP-SMX users appeared to perform substantially better (mean GDS, 0.29) than the 17 who did not report TMP-SMX use (mean GDS, 0.77) (P = .21; Figure 4). While our sample size is small and the difference not significant, the Cohen d value of 0.77 suggests that TMP-SMX might have a medium-to-large effect.
Figure 4.
Effect of trimethoprim-sulfamethoxazole (TMP-SMX) treatment on neurocognitive performance. Latent Toxoplasma infection–positive participants who reported TMP-SMX use (n = 3) performed substantially better than nonusers (n = 17), but the power of this comparison was low due to small numbers of participants. Heights of bars are means; lines are standard errors.
DISCUSSION
We investigated the effects of LTI on neurocognitive functioning, among people living with HIV disease and without a history or current signs or symptoms of Toxoplasma encephalitis, using a robust cognitive battery that assesses multiple domains. We found that LTI was associated with NCI, especially in those with higher CD4+ T-cell counts, but not with CSF biomarkers of inflammation. In a small subgroup of HIV+ patients, levels of anti-Toxo IgG correlated with NCI based on GDS.
The impact of LTI on brain functioning has been studied extensively in rodent models, a frequent mammalian intermediate host of T. gondii. Studies in humans have shown that persons with LTI have impaired reaction time [11] and are at a high risk of traffic accidents [12]. A recent population-based study reported impairment of memory in older adults (≥65 years) with LTI [10]. Their multivariate models evaluating only main effects demonstrated no association between LTI and cognitive function, but adding interactions revealed that LTI may decrease cognitive function in some demographic, educational, and ethnic/racial subgroups [17]. LTI is also associated with behavioral changes such as impulsivity and aggression [7] and increased suicide attempts [8].
The prevalence of LTI in the US population ranges from 9.5% to 13.2% [18, 19], and our T. gondii IgG positivity rate of 11.4% is consistent with these findings. Advanced immunosuppression due to HIV can lead to Toxoplasma encephalitis, a clinically obvious, progressive, and sometimes fatal opportunistic disease. Elevated HIV-related morbidity and mortality in LTI patients could falsely lower cross-sectional estimates of LTI prevalence. However, the facts that markers of HIV disease did not differ in those with and without LTI and that symptomatic Toxoplasma infections are relatively rare in the United States in the era of ART is evidence against such an effect. Although increased LTI in HIV-infected vs HIV-uninfected individuals has been reported [20, 21], other studies conducted in the United States, a low-prevalence country, and in Ethiopia, a very high-prevalence country, have found no differences [18, 22]. Thus, the prevalence of LTI in the general population is similar to the prevalence of LTI in the HIV population, consistent with the concept that HIV does not influence the rate of acquisition of LTI.
The LTI+ group was more likely to have undetectable levels of HIV RNA in blood compared with the LTI– group. Thus, they are less likely to have HIV-induced cognitive impairment based on inadequate treatment of their HIV infections. Our LTI+ group tended to be older, although not statistically significantly so, than the LTI– group, consistent with an expected age-related increase in prevalence of T. gondii infection [4, 19]. Age is unlikely to have confounded our analysis because scores on cognitive tests were adjusted for age. Because 90% of our study population were men, we were unable to determine an association between LTI and sex, but in the literature, sex as well as race/ethnicity and country of birth have not been found to increase LTI risk among HIV+ persons [19]. In contrast, in the general population, the risk of LTI is higher among non-Hispanic blacks and Mexican Americans than non-Hispanic whites, foreign-born than US-born persons, and those below the poverty index [23].
In the brains of latently infected mice and in tissue culture systems, toxoplasmic bradyzoites (T. gondii cysts) commonly rupture releasing tachyzoites that subsequently infect new neurons [24, 25]. Although normal immune responses virtually always contain the parasites, these reactions and consequent inflammation may damage the brain and degrade cognition. A cycle of reactivation and reinfection of new neurons may result in host responses that cumulatively damage and deplete neurons in both HIV-infected and -uninfected persons. This cycle could be enhanced in HIV patients during immune recovery on ART.
LTI appeared to be unrelated to nadir or current CD4+ T-cell count. However, in modeling NCI as a function of CD4+ T-cell count (Figure 2), we found that current levels of immune competence (CD4+ T-cell counts) had highly divergent effects on the probability of NCI in LTI+ and LTI– HIV-infected persons. In LTI– participants, the probability of NCI increases with lower CD4+ T-cell counts, consistent with prior findings [26]. The probability of NCI in LTI+ persons was higher overall and, in contrast to LTI– individuals, increased with higher CD4+ T-cell counts. We hypothesize that LTI+ individuals may incur subclinical brain damage because their immune reactions to toxoplasmic tachyzoites released from cysts is increased during immune reconstitution induced by ART. Our observation that anti-Toxo IgG levels correlate with NCI and with higher CD4+ T-cell counts is consistent with this hypothesis. During treatment-enabled immune recovery, improving IgG responses to toxoplasmic antigens could raise their blood levels. Thus, higher anti-Toxo IgG levels may reflect greater exposure to T. gondii antigen—that is, more frequent or substantial reactivations of LTI during periods of low CD4+ T-cell counts, or more robust antibody responses after ART-induced immune reconstitution, or both.
Because multiple biomarkers are associated with HAND [27], we measured biomarker levels in CSF where they might reflect inflammatory reactions to T. gondii in the brain better than blood levels. However, no differences between LTI+ and LTI– groups were seen in our panel of 4 biomarkers that are indicative of HIV-related neuroinflammation. Possible explanations include that (i) T. gondii leads to brain injury via mechanisms not reflected by these biomarkers; (ii) our cross-sectional study did not capture the brief periods after T. gondii cysts had ruptured and provoked inflammation; or (iii) power was limited by the small number of LTI+ persons analyzed (n = 5). A larger prospective study with frequent CSF collection could better address this issue.
LTI may be a treatable cause of persistent NCI in antiretroviral-treated, HIV-infected patients, although no evidence for the efficacy of drugs in suppressing subclinical reactivation of or clearing LTI is currently available. Older drug combinations such as TMP-SMX and pyrimethamine-sulfadiazine that are used to treat or prevent Toxoplasma encephalitis and newer drugs such as endochin-like quinolones may be effective in treating LTI [28]. LTI treatment could be initiated as soon as it is diagnosed in HIV patients and continued until complete ART-enabled immune reconstitution. Current guidelines support this practice for prevention of Pneumocystis pneumonia, Toxoplasma infections, and malaria in HIV adults with CD4+ T-cell counts <200 cells/µL. Prevention of LTI-induced NCI may be an additional benefit, especially in low-income countries, which generally have higher prevalence of LTI.
Limitations of this study include (i) lack of longitudinal data, (ii) lack of HIV-uninfected controls, and (iii) a small number of participants for subgroup analyses (eg, quantification of anti-Toxo IgG levels in serum, CSF biomarkers, and TMP-SMX users).
CONCLUSIONS
In this cross-sectional analysis of HIV-infected, predominately antiretroviral-treated patients, LTI was associated with risk of NCI, especially in those with CD4+ T-cell counts >200 cells/µL. Higher levels of CD4+ T cells and of anti-Toxo IgG also correlated with NCI, possibly reflecting damage from improved immune responses to subclinical reactivation of Toxoplasma in brain during ART.
Longitudinal studies of patients before and after ART initiation could test our hypothesis that immune reconstitution plays a role in the pathogenesis of cognitive impairment in patients with LTI. Interventional trials with existing or investigational drugs active against Toxoplasma cysts would further strengthen our findings and could lead to new treatments for HAND in people living with LTI.
Notes
Financial support. This work was supported by the following grants from the National Institutes of Health: A. R. B. (K23 MH085512, R01 DA039775); D. M. S. (R01 MH097520, K24 AI100665); S. L. L. (K24 MH097673); National Institute on Drug Abuse (P01 DA12065, R01 DA16015); and National Institute of Mental Health (P30 MH62512).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Heaton RK, Marcotte TD, Mindt MR et al. . The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 2004; 10:317–31. [DOI] [PubMed] [Google Scholar]
- 2.Letendre SL, Bharti AR, Perez-Valero I et al. . Higher cytomegalovirus antibody concentrations are associated with older age, lower nadir CD4+ cell counts, and worse global neurocognitive functioning in people with HIV disease. In: 19th Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 2012. [Google Scholar]
- 3.Sun B, Abadjian L, Rempel H, Monto A, Pulliam L. Differential cognitive impairment in HCV coinfected men with controlled HIV compared to HCV monoinfection. J Acquir Immune Defic Syndr 2013; 62:190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones JL, Kruszon-Moran D, Rivera HN, Price C, Wilkins PP. Toxoplasma gondii seroprevalence in the United States 2009–2010 and comparison with the past two decades. Am J Trop Med Hyg 2014; 90:1135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyons RE, McLeod R, Roberts CW. Toxoplasma gondii tachyzoite-bradyzoite interconversion. Trends Parasitol 2002; 18:198–201. [DOI] [PubMed] [Google Scholar]
- 6.Abgrall S, Rabaud C, Costagliola D; Clinical Epidemiology Group of the French Hospital Database on HIV. Incidence and risk factors for toxoplasmic encephalitis in human immunodeficiency virus-infected patients before and during the highly active antiretroviral therapy era. Clin Infect Dis 2001; 33:1747–55. [DOI] [PubMed] [Google Scholar]
- 7.Coccaro EF, Lee R, Groer MW, Can A, Coussons-Read M, Postolache TT. Toxoplasma gondii infection: relationship with aggression in psychiatric subjects. J Clin Psychiatry 2016; 77:334–41. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen MG, Mortensen PB, Norgaard-Pedersen B, Postolache TT. Toxoplasma gondii infection and self-directed violence in mothers. Arch Gen Psychiatry 2012; 69:1123–30. [DOI] [PubMed] [Google Scholar]
- 9.Hodkova H, Kodym P, Flegr J. Poorer results of mice with latent toxoplasmosis in learning tests: impaired learning processes or the novelty discrimination mechanism? Parasitology 2007; 134:1329–37. [DOI] [PubMed] [Google Scholar]
- 10.Gajewski PD, Falkenstein M, Hengstler JG, Golka K. Toxoplasma gondii impairs memory in infected seniors. Brain Behav Immun 2014; 36:193–9. [DOI] [PubMed] [Google Scholar]
- 11.Havlicek J, Gasova ZG, Smith AP, Zvara K, Flegr J. Decrease of psychomotor performance in subjects with latent ‘asymptomatic’ toxoplasmosis. Parasitology 2001; 122:515–20. [DOI] [PubMed] [Google Scholar]
- 12.Flegr J, Klose J, Novotna M, Berenreitterova M, Havlicek J. Increased incidence of traffic accidents in Toxoplasma-infected military drivers and protective effect RhD molecule revealed by a large-scale prospective cohort study. BMC Infect Dis 2009; 9:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antinori A, Ammassari A, De Luca A et al. . Diagnosis of AIDS-related focal brain lesions: a decision-making analysis based on clinical and neuroradiologic characteristics combined with polymerase chain reaction assays in CSF. Neurology 1997; 48:687–94. [DOI] [PubMed] [Google Scholar]
- 14.Heaton RK, Clifford DB, Franklin DR Jr et al. . HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carey CL, Woods SP, Gonzalez R et al. . Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 2004; 26:307–19. [DOI] [PubMed] [Google Scholar]
- 16.Anderson AM, Harezlak J, Bharti A et al. . Plasma and cerebrospinal fluid biomarkers predict cerebral injury in HIV-infected individuals on stable combination antiretroviral therapy. J Acquir Immune Defic Syndr 2015; 69:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gale SD, Brown BL, Erickson LD, Berrett A, Hedges DW. Association between latent toxoplasmosis and cognition in adults: a cross-sectional study. Parasitology 2015; 142:557–65. [DOI] [PubMed] [Google Scholar]
- 18.Israelski DM, Chmiel JS, Poggensee L, Phair JP, Remington JS. Prevalence of Toxoplasma infection in a cohort of homosexual men at risk of AIDS and toxoplasmic encephalitis. J Acquir Immune Defic Syndr 1993; 6:414–8. [PubMed] [Google Scholar]
- 19.Mathews WC, Fullerton SC. Use of a clinical laboratory database to estimate Toxoplasma seroprevalence among human immunodeficiency virus-infected patients. Overcoming bias in secondary analysis of clinical records. Arch Pathol Lab Med 1994; 118:807–10. [PubMed] [Google Scholar]
- 20.Machala L, Maly M, Hrda S, Rozsypal H, Stankova M, Kodym P. Antibody response of HIV-infected patients to latent, cerebral and recently acquired toxoplasmosis. Eur J Clin Microbiol Infect Dis 2009; 28:179–82. [DOI] [PubMed] [Google Scholar]
- 21.Meisheri YV, Mehta S, Patel U. A prospective study of seroprevalence of toxoplasmosis in general population, and in HIV/AIDS patients in Bombay, India. J Postgrad Med 1997; 43:93–7. [PubMed] [Google Scholar]
- 22.Shimelis T, Tebeje M, Tadesse E, Tegbaru B, Terefe A. Sero-prevalence of latent Toxoplasma gondii infection among HIV-infected and HIV-uninfected people in Addis Ababa, Ethiopia: a comparative cross-sectional study. BMC Res Notes 2009; 2:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones JL, Kruszon-Moran D, Sanders-Lewis K, Wilson M. Toxoplasma gondii infection in the United States, 1999–2004, decline from the prior decade. Am J Trop Med Hyg 2007; 77:405–10. [PubMed] [Google Scholar]
- 24.Hermes G, Ajioka JW, Kelly KA et al. . Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J Neuroinflammation 2008; 5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watts E, Zhao Y, Dhara A, Eller B, Patwardhan A, Sinai AP. Novel approaches reveal that Toxoplasma gondii bradyzoites within tissue cysts are dynamic and replicating entities in vivo. MBio 2015; 6:e01155–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellis RJ, Badiee J, Vaida F et al. . CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 2011; 25:1747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcotte TD, Deutsch R, Michael BD et al. . A concise panel of biomarkers identifies neurocognitive functioning changes in HIV-infected individuals. J Neuroimmune Pharmacol 2013; 8:1123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doggett JS, Nilsen A, Forquer I et al. . Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis. Proc Natl Acad Sci U S A 2012; 109:15936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]