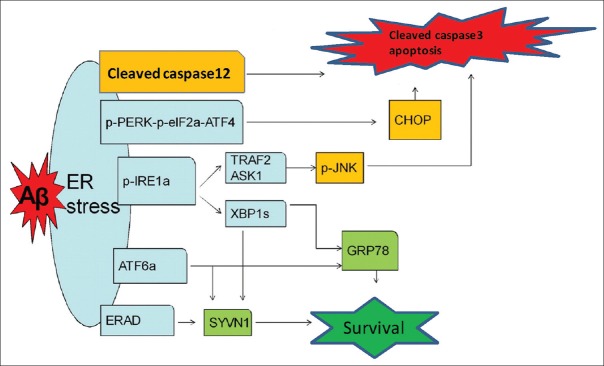
Figure 4.
Schematic drawing shows that activated UPR pathways of ERS induced by intraneuronal β-amyloid in 5×FAD mice. Aβ in 2-month-old 5×FAD brains lead to higher ERS level than that of WT mice, which produces a cellular protective effect, on the one hand, up-regulating the expression of ER chaperones and related degradation proteins mainly via p-IRE-1α-XBP1s and ATF6α pathways at the early stage of AD to eliminate Aβ; on the other hand, inducing increased expression of downstream signaling pathways molecules, especially pro-apoptotic proteins (cleaved caspase-12 and CHOP). Under sustained stress conditions caused by the Aβ-associated toxic effects, the protective function of the UPR declines and the pro-apoptotic functions are enhanced gradually. The pro-apoptotic functions of the UPR may lead to functional impairment of neurons and even death. UPR: Unfolded protein response; ERS: Endoplasmic reticulum stress; Aβ: Amyloid β; 5×FAD: Transgenic mice with five familiar Alzheimer's disease; p-PERK: Phosphorylated protein kinase RNA-like ER kinase; p-eIF2α: Phosphorylated eukaryotic translation initiation factor 2α; ATF4: Activating transcription factor 4; CHOP: CCAAT/enhancer-binding protein homologous protein; p-IRE-1α: Phosphorylated inositol-requiring enzyme 1α; XBP1s: Spliced X box-binding protein 1; ATF6α: Activating transcription factor 6α; GRP 78: Glucose-regulated protein 78; ERAD: Endoplasmic reticulum-associated protein degradation; SYVN1: Ubiquitin ligase synovial apoptosis inhibitor 1; TRAF2: Tumor necrosis factor-receptor-associated factor 2; ASK1: Apoptosis signal-regulating kinase 1; JNK: c-Jun amino-terminal kinase.