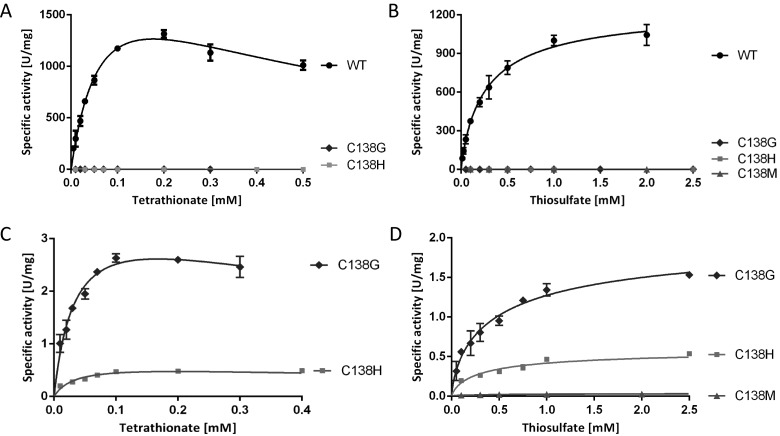
Figure 3. Enzyme activity assays with TsdA wt and Haem 1 ligation affected variants.
TsdA wt was compared with TsdA C138G, C138H and C138M concerning tetrathionate reduction (A) and thiosulfate oxidation (B). Tetrathionate reduction (C) and thiosulfate oxidation (D) of the TsdA C138 variants are shown in detail in the panels below. Tetrathionate reduction (A and C) was measured under anoxic conditions at 42°C in 100 mM ammonium acetate buffer (pH 5) with 0.3 mM methyl viologen previously reduced with titanium (III) citrate. Control measurements showed that methyl viologen was provided at a saturating concentration in all cases. Tetrathionate reduction activity of TsdA C138M was so low that exact kinetic parameters could not be derived with confidence. Different tetrathionate concentrations (0.01–0.7 mM) were used. Thiosulfate oxidation (B and D) was measured at 42°C in 50 mM BisTris buffer (pH 6.5) with 80 μM horse heart cytochrome c as electron acceptor and 0.05–8 mM thiosulfate. Control measurements showed that horse heart cytochrome c was provided at a saturating concentration in all cases.