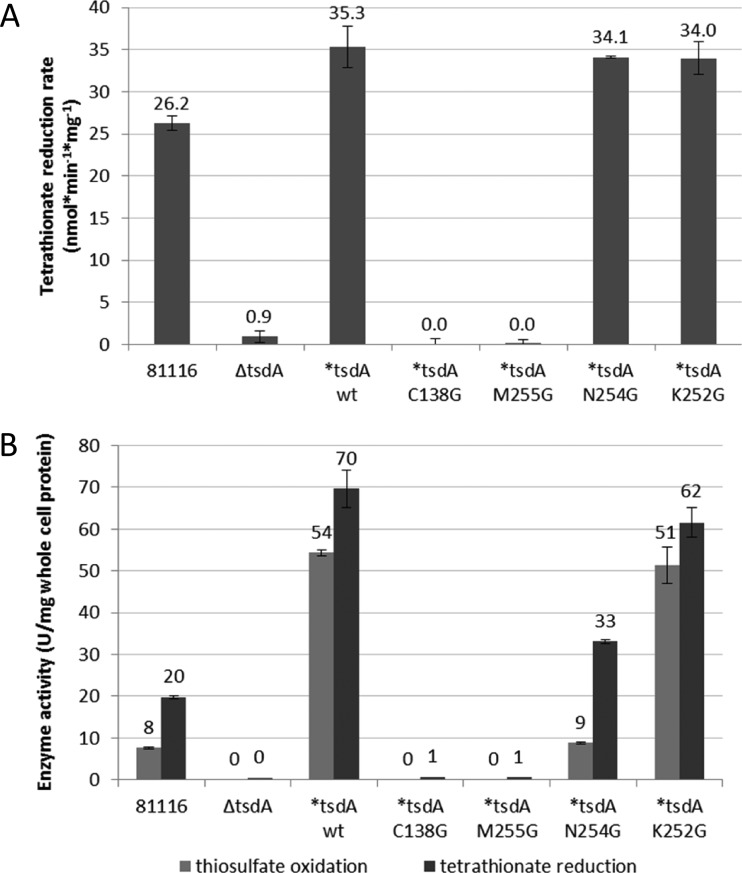
Figure 8. Determination of tetrathionate reduction rate with whole cells and enzyme activity assays with crude cell extract.
For determination of tetrathionate reduction rate with whole cells (A) C. jejuni cells of 81116 wt (81116), ΔtsdA mutant (ΔtsdA) and the different complementation strains (*tsdA WT, *tsdA C138G, *tsdA M255G, *tsdA N254G, *tsdA K252G) were incubated at 42°C in 14 ml phosphate buffer (pH 7.4) containing 20 mM sodium formate and 2 mM tetrathionate. The thiosulfate concentrations in the supernatants of samples taken at 2 or 5 min intervals were determined directly after the experiment. For enzyme activity assays with crude cell extract (B) C. jejuni cells of 81116 wt (81116), ΔtsdA mutant (ΔtsdA) and the different complementation strains (*tsdA WT, *tsdA C138G, *tsdA M255G, *tsdA N254G, *tsdA K252G) were disrupted by bead beating. Tetrathionate reduction was measured anaerobically at 42°C in 100 mM ammonium acetate buffer (pH 5) with 0.3 mM methyl viologen previously reduced with titanium (III) citrate and 0.1 mM tetrathionate. Thiosulfate oxidation was performed at 42°C in 50 mM BisTris buffer (pH 6.5) with 80 μM horse heart cytochrome c as electron acceptor and 2 mM thiosulfate.