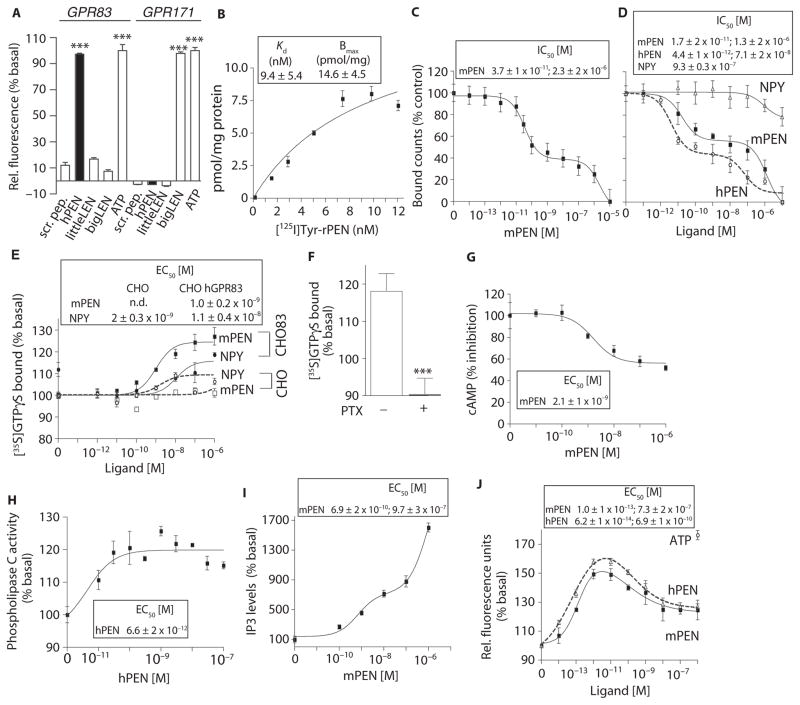
Fig. 4. Expression of GPR83 in heterologous cells confers PEN binding and signaling.
(A) The effect of PEN (1 μM) on intracellular Ca2+ release in cells expressing hGPR83 along with a promiscuous chimeric hGα16/i3 protein. Scrambled peptide (1 μM), hPEN (1 μM), rlittleLEN (1 μM), mbigLEN (1 μM), or ATP (1 μM). (B) Saturation binding with [125I]Tyr-rPEN in CHO hGPR83 cell membranes (30 μg). (C) mPEN displacement of [125I]Tyr-rPEN (3 nM) binding to HEK-293 mGPR83 cells (50 μg). (D) The ability of mPEN, hPEN, and NPY to displace [125I]Tyr-rPEN (3 nM) binding to CHO hGPR83 cell membranes (50 μg). (E) The effect of mPEN on GTPγS binding to membranes (20 μg) from CHO hGPR83 cells (CHO83) or CHO alone. (F) The effect of pertussis toxin (PTX; 50 ng/ml) pretreatment on 1 μM PEN-mediated [35S]GTPγS binding in membranes (20 μg) from CHO hGPR83 cells versus absence of PTX. ***P < 0.0005 (t test). (G) The effect of mPEN on intracellular cAMP levels in CHO hGPR83 cells (10,000 cells per well). (H) The effect of PEN on PLC activity in membranes (10 μg) from CHO hGPR83 cells. (I) The effect of mPEN on IP3 levels in CHO hGPR83 cells (10,000 cells per well). (J) The effect of mPEN or hPEN on intracellular Ca2+ release in CHO hGPR83 cells expressing a promiscuous chimeric hGα16/i3 protein. ATP (1 μM) was used as a positive control. Data (A to J) represent means ± SE (n = 3 to 8 independent experiments). ***P < 0.001[one-way ANOVA for (A); t test for (F)]; details of the statistical analyses are in table S1.