TABLE 3.
Population pharmacokinetic parameter estimates of indinavira
| Parameter | Population mean
|
Interindividual variabilityb
|
|||
|---|---|---|---|---|---|
| Estimate | 95% CI | % SEc | % Estimate | % SEc | |
| CLfemale (liters/h) | 32.4 | 27.8-37.2 | 13.5 | 39.0 | 44.8d |
| θmalee | 0.30 | 0.14-0.49 | 56.5 | ||
| θritonavirf | −0.63 | −0.6 to 0.64 | 6.5 | ||
| θBWg | 0.16 | 0.12-0.35 | 44.8 | ||
| V (liters) | 65.7 | 55.9-76.0 | 13.0 | ||
| Ka (h−1) | 1.0 | 0.80-1.41 | 21.8 | 67.0 | 65.5d |
| F | 1.0h | — | — | ||
| σ (CV%)i | 41 | 51.7d | |||
| σ (SD; μg/liter)j | 670 | 56.6 | |||
Final model for clearance: CL = CLfemale·(1 + θmale)·(1 + θritonavir)·[1 + θBW·(BW − 70)/70], with ritonavir, and sex (female or male) expressed as index variables.
Estimates of variability expressed as CV%.
SE, standard error of the estimates, expressed as CV%.
SE, standard error of the variance components, taken as 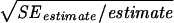 , expressed as a percentage.
, expressed as a percentage.
Relative increase in CL in male patients, compared to female patients.
Relative decrease in CL in presence of ritonavir.
Proportionality term relating CL to a relative increase or decrease in body weight (kg) from the average population value of 70 kg.
Set to 1 because intravenous drug administration not available, hence no 95% CI or % SE evaluable (—).
Residual variability in the plasma concentrations.
Additive component of the residual variability in the plasma concentration.
