Figure 4. Establishment of iPSC-based SPG4 and SPG3A models that recapitulate disease-specific axonal phenotypes.
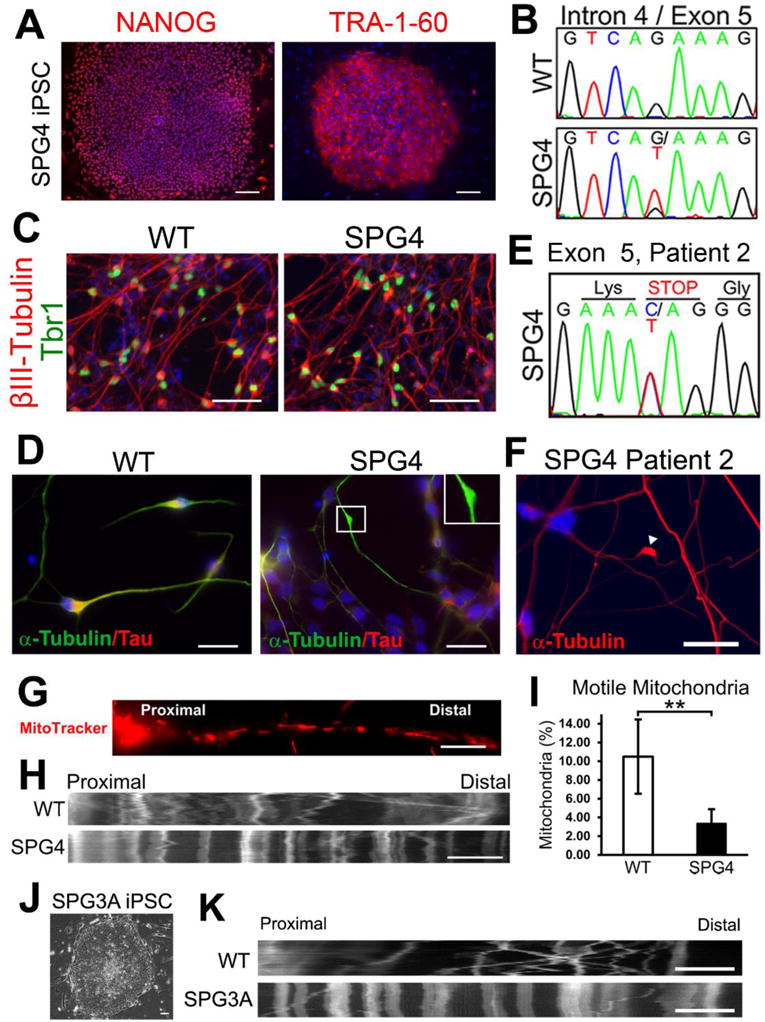
(A, B) Immunostaining showing the expression of pluripotent protein NANOG and TRA-1-60 (A) by the iPSCs derived from a patient with intron 4 splice acceptor mutation (c.683-1G>T; panel B). (C) At 6 weeks after differentiation, telencephalic glutamatergic neurons (Tbr1+/βIII-tubulin+) were efficiently generated from WT (control) and SPG4 iPSCs. (D) Neurons derived from SPG4 iPSCs displayed swellings in Tau+ axons, while control neuron axons were mostly smooth with no swellings. (E, F) Increased formation of axonal swellings was also observed from telencephalic neurons derived from iPSCs of another patient with a C>T transition located in Exon 5 of the SPAST gene (amber mutation, E). (G) To examine fast axonal transport of mitochondria, cells were stained with MitoTracker Red CMXRos (Invitrogen). (H) Representative distance versus time kymographs over a 5 minute recording. (I) Quantification of motile mitochondria in week 8 telencephalic neurons showed a significant decrease of motile mitochondria in SPG4 neurons compared to control neurons. Data presented as mean ± SD. **P < 0.01. (J) SPG3A fibroblast cells were successfully programmed to iPSCs that have typical ESC morphology. (K) As shown by the representative distance versus time kymographs, reduction of motile mitochondria was also observed in SPG3A iPSC-derived telencephalic neurons. Blue indicates Hoechst stained nuclei. Bars, 100 (A), 50 (C), 20 (D,F), 10 (G, H), and 5 (K) μm. Modified from references (Denton et al., 2014; Zhu et al., 2014).
